Figure 1.
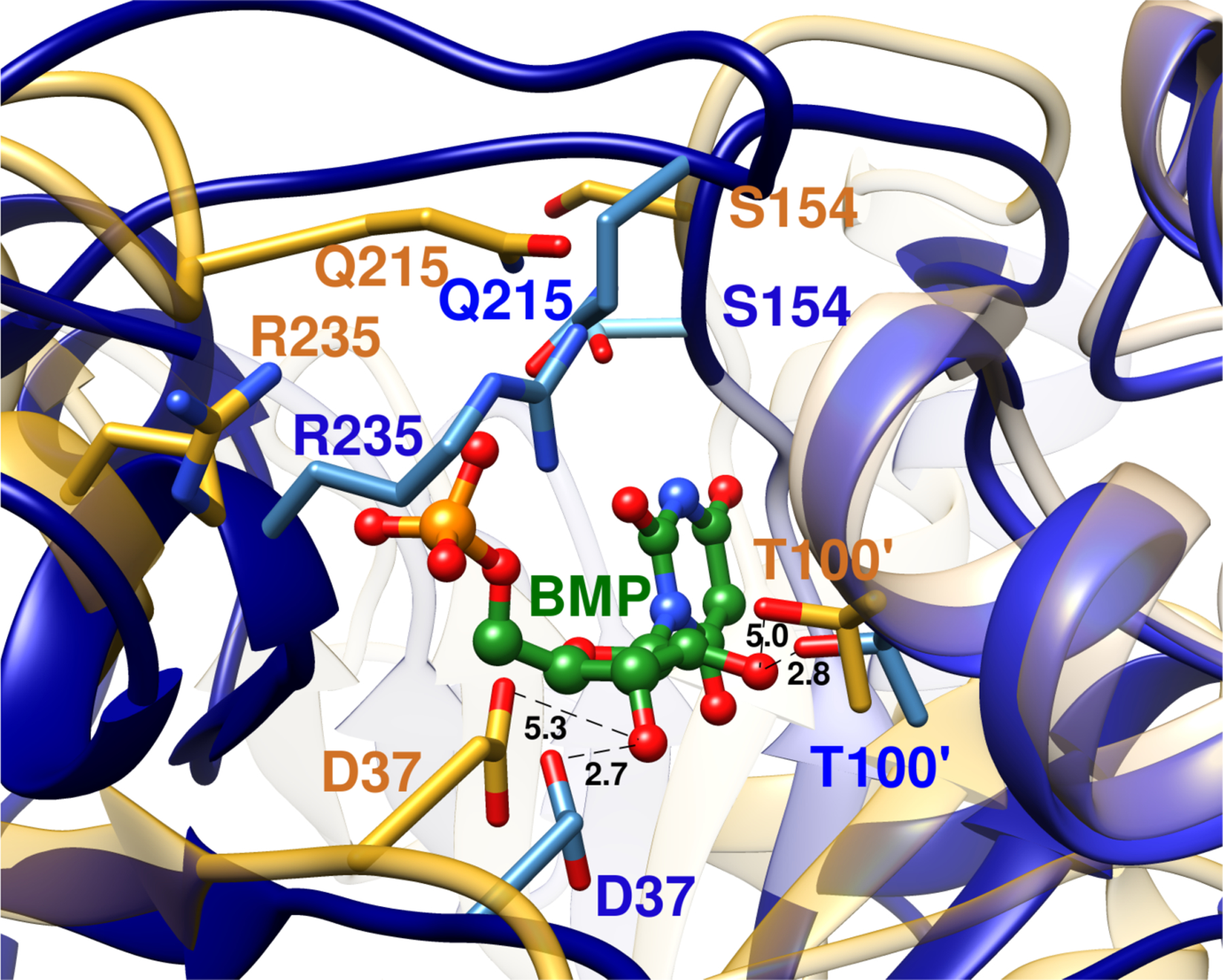
Superposition of representations of the X-ray crystal structure of the unliganded open form of yeast OMPDC (gold, PDB 1DQW) and of the closed enzyme (blue, PDB 1DQX) complexed to the tight binding inhibitor 6-hydroxyuridine 5’-monophosphate (BMP, green). The inhibitor complex is stabilized by movement of Q215 and R235 side chains towards the ligand phosphodianion, and of the S154 side chain at a pyrimidine umbrella loop into a position to hydrogen bond to the Q215 side chain. The D37 and T100’ side chains are shown in gold for unliganded OMPDC. They are an estimated 5.3 Å and 5.0 Å, respectively, from the C-3’-OH and C-2’-OH ribosyl-OH at the hypothetical BMP ligand, which is superimposed with BMP bound to the closed enzyme. The D37 and T100’ side chains are shown in blue for the closed BMP complex. They lie 2.7 Å and 2.8 Å, respectively, from the C-3’-OH and C-2’-OH groups of the ligand.
