Abstract
Background
Studies suggest a high prevalence of pain in head and neck cancer (HNC) patients at diagnosis, during and after treatment; however, these studies had small sample sizes and did not comprehensively assess factors known to influence pain. We surveyed a large cohort of HNC survivors to determine variations in the prevalence of pain, its treatment and management by duration of survivorship, and assessed a comprehensive list of risk factors.
Methods
A cross sectional survey of post-treatment survivors of HNC during routine follow-up clinic visits.
Results
A total of 505 HNC survivors with a median follow up of 3 years from cancer diagnosis were included in the study. Overall, 45% (n = 224) reported pain and 14.5, 22 and 7% reported use of prescribed pain medication, over-the-counter pain medication and alternative pain therapies, respectively. Prevalence of severe pain was 7.3% and did not vary significantly by years of survivorship (< 1 year = 5.7%; 1 to < 3 years = 7.1%; 3 to < 8 years = 7.6%; 8 years or more =9.7%; P = 0.392). However, use of prescribed pain medication significantly varied by years of survivorship (< 1 year = 45.7%; 1 to < 3 years = 24.6%; 3 to < 8 years = 18.9; 8 years or more = 18.3%; p < 0.001). Of note, a significant proportion of survivors reported moderate to severe pain (moderate to severe = 55.7% versus none to mild = 44.3%) despite step 3 analgesic use (p < 0.001). Multivariable regression shows that recurrent disease (OR 6.77, 95% CI [1.44, 31.80]), history of chemotherapy (OR 6.00, 95% CI [2.10, 17.14]), and depression (Mild-moderate OR 5.30, 95% CI [2.20, 12.78]; Major OR 8.00, 95% CI [2.67, 23.96]) were significant risk factors for severe pain.
Conclusions
We identified a high prevalence of pain among HNC survivors and determined that analgesic use varied by the duration of survivorship. Therefore, routine surveillance for pain must be consistent throughout the course of survivorship.
Keywords: Pain, Head and neck cancer, Survivorship, Opioids
Background
An individual is considered a cancer survivor from the time of diagnosis until the end of life [1]. For many cancer patients, the survivorship period comes with a variety of challenges, including the management of symptoms that persist beyond the completion of cancer treatment [2–7]. Pain is perhaps one of the most distressing symptoms of head and neck cancer (HNC) patients at diagnosis [2, 8]. Pain may also arise as a complication or toxicity of HNC treatment including surgery, radiotherapy or chemotherapy. Standard treatment of HNC is based largely on primary tumor location and cancer stage. Early stage disease is treated with single modality treatment and advanced stage disease is treated with multi-modal therapy [9].
HNC accounts for 3% of all cancer survivors in the United States, with long-term survival in this population becoming more common due to improved treatment modalities. About 80 to 90% of early stage patients enter remission, and HPV-related HNC is associated with a significantly better prognosis relative to other cancers, with cure rates approaching 90% [9]. With improving survival rates, the American Cancer Society guidelines for HNC survivors underscore the need to recognize the potential late and long-term complications or toxicities of cancer treatment, as well as its under-treatment and management [9]. While most studies suggest the high prevalence of pain in HNC patients at diagnosis, during treatment and post-treatment, these studies have small samples of patients and do not include a comprehensive assessment of factors known to influence pain [6, 10–13].
In this study, we surveyed a large cohort of HNC survivors to determine variations in the prevalence of pain, its treatment and management by duration of survivorship, and assessed a comprehensive list of risk factors. We first focused on the relationship between pain and reports of analgesic use by years of survivorship. We also determined the epidemiological, clinical and behavioral variables associated with pain. Given that pain in HNC patients have been shown to influence survival and quality of life, understanding the epidemiology of pain during the survivorship period in HNC survivors has huge clinical significance [6, 10–13].
Methods
Study population
HNC patients were recruited during their scheduled follow-up clinic visits between May 1, 2013 to January 31, 2017 at the Head and Neck Center of The University of Texas MD Anderson Cancer Center (MDACC) in Houston, Texas, USA. Eligibility criteria included patients who were ≥ 18 years of age, able to speak English or Spanish, with biopsy-confirmed squamous cell carcinoma of the head and neck and have completed cancer treatment at MDACC. All procedures involving human subjects were conducted in accordance with ethical guidelines and regulatory approval from the Institutional Review Board of MDACC. Research staff administered standardized questionnaires, and participants who could not complete the study on-site had the option to submit questionnaires by mail using prepared envelopes. The response rate was 79.3%.
Study variables
Pain severity was assessed with “Have you had pain in the past week? If yes-please indicate how bad your pain has been in the last week by marking an X (on an 11-point visual analog scale) from 0 (no pain) to 10 (as severe as it could be)” [14]. Responses were categorized according to the 2019 guidelines of the National Comprehensive Cancer Network as absence of pain (0), mild pain (score of 1 to 3), moderate pain (score of 4 to 7), and severe pain (score of 8 to 10) [15, 16]. Using an illustrated body map, patients were asked to indicate pain locations, which were subsequently categorized by RDR: (1) head and oral cavity, (2) neck and throat, (3) shoulder, (4) upper extremities, (5) anterior chest, (6) posterior chest, (7) abdomen, (8) lower back and pelvis, and (9) lower extremities.
Socio-demographic information (age, sex, marital status, education, employment, ethnicity/race), cancer history (location, recurrence, other primary cancer), history of cancer treatment (chemotherapy, radiotherapy, or surgery), and comorbidities were collected by self-report. Information regarding cancer history and treatment was supplemented by a review of electronic medical records. Comorbidities included a prior diagnosis of pain-related conditions (osteoarthritis, neuropathic pain, herniated disk and radiculopathy) and hypertension, diabetes and coronary artery disease.
The use of pain medication was assessed with the questions “Have you taken prescription medication for your pain during the past 3 months?”, “If yes, please specify name of medication, dose and frequency and dates you started and stopped them. Dose refers to the amount of medicine and is usually marked on the pill bottle. Example: 1 teaspoon, 30cc, 5 mg”; “Have you taken over-the-counter medication for your pain, during the past 3 months?” and “If yes, please specify name of medication, dose and frequency and dates you started and stopped them. Dose refers to the amount of medicine and is usually marked on the pill bottle. Example: 1 teaspoon, 30 cc, 5 mg”. Using the World Health Organization (WHO) analgesia ladder, RDR categorized the responses accordingly: Step 0 for no analgesia, Step 1 for non-opioid pain medication, Step 2 for weak opioids, or Step 3 for strong opioids [17].
Depression risk was assessed using the Community Epidemiologic Studies Depression Scale (CES-D) [18], a 20-item self-report questionnaire that is widely used in research studies to screen for psychological distress (symptoms of depression and anxiety). Each item was scored on a 4-point Likert scale (0 = rarely, 3 = most of the time), yielding a maximum score of 60. A CES-D score of 16 to 26 is indicative of mild to moderate risk of depression, while a score of 27 or higher suggests major risk [19]. All data were recorded using Research Electronic Data Capture (REDCap) [20].
Statistical analysis
Demographic, clinical, and behavioral characteristics of the study population were summarized using descriptive statistics. We used a Pearson chi-square test to compare categorical variables and Fisher’s exact test when the expected count was < 5. Student’s t-test was used to compare continuous variables.
We conducted logistic regression to assess variables associated with pain. Variables with a P value of ≤0.2 were considered candidates for multivariable logistic regression analysis to evaluate risk factors of severe pain in HNC survivors. A P value of 0.20 was used as the cutoff because using a more traditional level (P < 0.05) often fails to identify variables known to be important [21]. Patients with complete information on the candidate variables were used in the analyses. All statistical analyses were performed using IBM Statistical Package for the Social Sciences version 24 (SPSS Inc., Chicago, IL). An alpha (α) level of 0.05 was considered significant.
Results
A total of 637 patients were approached to participate in the study with 561 consenting to participate. However, 56 were excluded after enrollment because they did not meet all the pre-specified inclusion criteria (after further review of pathological reports). A common reason for non-participation was their busy clinic schedule. A total of 505 HNC survivors were included in the analysis. The demographic and clinical characteristics of this population are summarized in Table 1. The mean and median ages were 61.5 (SD: 10.6) and 62 (range: 19–93) years, respectively. A majority were male (n = 392; 76.6%), married or cohabiting (n = 405; 80.4%) and non-Hispanic White (n = 457; 90.5%). The mean duration of survivorship was 4.6 (SD: 4.3) years from diagnosis and a median duration of 3 years (range = 1–36). Cancers of the oropharynx (53.5%) and oral cavity (23.0%) were the most common, which is consistent with contemporary prevalence data [22]. History of cancer treatment included receipt of radiotherapy (81.5%), surgery (62.9%) and chemotherapy (58.1%). The most common comorbid conditions were hypertension (43.4%), herniated disc and radiculopathy (22.1%), osteoarthritis (13%), and a prior diagnosis of neuropathic pain (11.6%).
Table 1.
Characteristics of the study population (N = 505)
| Characteristics | N | (%) |
|---|---|---|
| Age (years) | ||
| Mean (Std.Dev) | 61.5 | (10.6) |
| Median (Min-Max) | 62 | (19–93) |
| Sex | ||
| Male | 392 | (77.6) |
| Female | 113 | (22.4) |
| Marital Status | ||
| Single or separated | 99 | (19.6) |
| Married or cohabiting | 405 | (80.4) |
| Not available | 1 | |
| Education | ||
| No college degree | 225 | (44.9) |
| College graduate | 276 | (55.1) |
| Not available | 4 | |
| Employment | ||
| Not employed or retired | 256 | (51.0) |
| Employed | 246 | (49.0) |
| Not available | 3 | |
| Race/Ethnicitya | ||
| Non-Hispanic White | 457 | (90.5) |
| Other | 48 | (9.5) |
| Survivorship (years)b | ||
| Average (St.Dev) | 4.6 | (4.3) |
| Median (Min-Max) | 3 | (1–36) |
| Survivorship (quartiles)b | ||
| ≤ 1 year | 141 | (27.9) |
| > 1 to ≤3 years | 115 | (22.8) |
| > 3 to ≤8 years | 145 | (28.7) |
| > 8 years | 104 | (20.6) |
| Site of HNC | ||
| Oropharynx | 270 | (53.5) |
| Oral cavity | 116 | (23.0) |
| Larynx | 55 | (10.9) |
| Other | 64 | (12.7) |
| Recurrent disease | 85 | (16.9) |
| Other primary cancer | 72 | (14.3) |
| Treatmentc | ||
| Chemotherapy | 293 | (58.1) |
| Radiotherapy | 411 | (81.5) |
| Surgery | 317 | (62.9) |
| Modality | ||
| Single | 133 | (26.3) |
| Two | 223 | (44.2) |
| Three | 147 | (29.2) |
| Comorbiditiesc | ||
| Hypertension | 213 | (43.4) |
| Herniated disc and radiculopathy | 109 | (22.1) |
| Osteoarthritis | 64 | (13.0) |
| Neuropathic pain | 57 | (11.6) |
| Diabetes | 55 | (11.2) |
| Coronary heart disease | 47 | (9.6) |
| Emphysema and lung disease | 31 | (6.3) |
| Rheumatoid arthritis | 31 | (6.3) |
| Cerebrovascular disease | 21 | (4.3) |
| Pain Status | ||
| With Pain | 227 | (45.0) |
| No Pain | 278 | (55.0) |
| Pain Intensityd | ||
| No Pain | 278 | (55.4) |
| Mild | 89 | (17.7) |
| Moderate | 98 | (19.5) |
| Severe | 37 | (7.4) |
| Not available | 3 | |
| Using pain medication | ||
| Yes | 147 | (29.1) |
| No | 355 | (70.3) |
| Not available | 3 | |
| Using prescription pain medication | 73 | (14.5) |
| Using weak opioids | 7 | (1.4) |
| Using strong opioids | 70 | (13.9) |
| Using over-the-counter pain medication | 112 | (22.2) |
| Using psychotropic medication for pain | 19 | (3.8) |
| Using any alternative pain therapies | 39 | (7.7) |
| Analgesia Classificatione | ||
| No analgesic | 355 | (70.4) |
| Step 1 | 76 | (15.1) |
| Step 2 | 3 | (0.6) |
| Step 3 | 70 | (13.9) |
| Not available | 1 | |
aRace categories included: White; Other = Black, African American, or African; American Indian/Alaska Native; Native Hawaiian or Other Pacific Islander; Asian; Other (specify)
Ethnicity included: non-Hispanic; Hispanic
bNumber of years from date of HNC diagnosis
cCategories are non-exclusive. Displayed is “yes” response to exposure/having comorbidity
dBased on NCCN 2019 cancer pain criteria
eWorld Health Organization analgesia ladder
Prevalence, severity, and management of pain
As many as 45% of HNC survivors reported pain (Table 1). Using the National Comprehensive Cancer Network (NCCN) cancer pain severity categories [16], we found that 7.4% (n = 37) HNC survivors had severe pain while 19.5% (n = 98) and 17.7% (n = 89) had moderate and mild pain, respectively. Based on self-report, 14.5% (n = 73) had prescriptions for pain medication and 22.2% (n = 112) reported using over-the-counter pain medication. The most frequently reported locations of pain (Table 2) were in the regions of the neck and throat (25.5%), head and oral cavity (14.7%), and shoulder (6.8%).
Table 2.
Pain sites among HNC survivors with pain (n = 251)
| Location | Frequencya |
|---|---|
| Head and Oral Cavity | 14.7% |
| Neck and Throat | 25.5% |
| Shoulder | 6.8% |
aReported values are frequency of site reported as location of pain by HNC survivors
Based on WHO analgesia ladder, 15% were on Step 1 analgesics including acetaminophen and non-steroidal anti-inflammatory drugs (NSAIDs). Only 0.6% (n = 3) were on Step 2 analgesics which include the use of a weak opioid with or without NSAIDs or adjuvant medication, and 13.9% (n = 70) were on Step 3 analgesics. Additionally, 7.7% (n = 39) of participants reported alternative pain treatment such as acupuncture and massages.
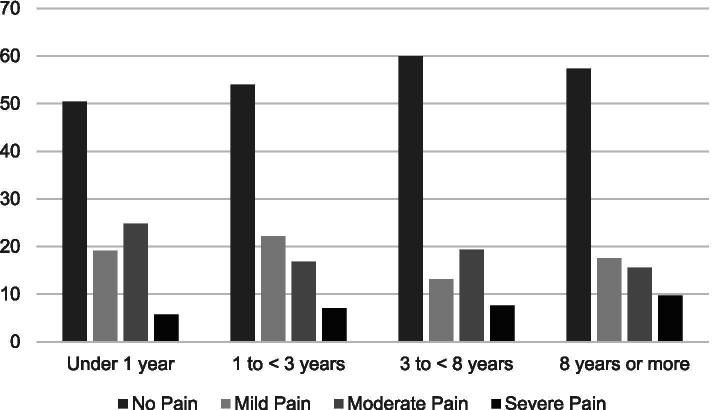
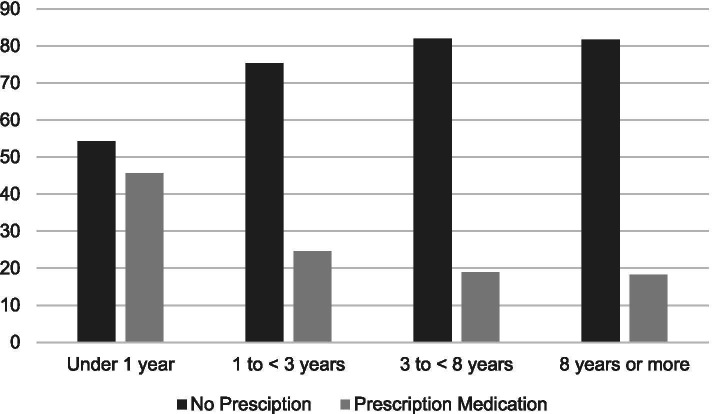
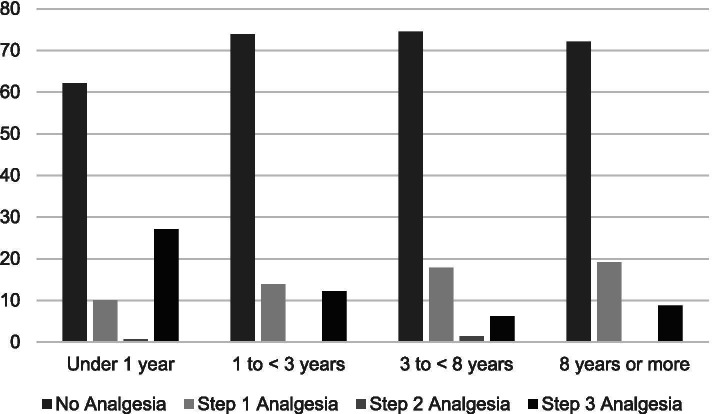
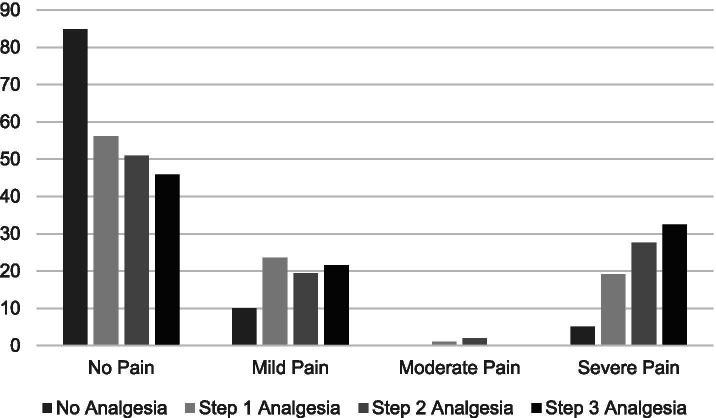
Stratified by years of survivorship, Fig. 1 shows that pain severity ratings did not vary significantly by years of survivorship (< 1 year = 5.7%; 1 to < 3 years = 7.1%; 3 to < 8 years = 7.6%; 8 or more years = 9.7%; P = 0.392). Figure 2 shows use of prescribed pain medication significantly varied by years of survivorship (< 1 year = 45.7%; 1 to < 3 years = 24.6%; 3 to < 8 years = 18.9; 8 years or more = 18.3%; p < 0.001). Figure 3 shows reports of opioid use by years of survivorship, with the use of strong opioids (Step 3) highest among those who are under 1 year from diagnosis. Figure 4 shows pain severity stratified by analgesic use. Of note, a significant proportion of survivors reported moderate to severe pain (moderate to severe = 55.7% versus none to mild = 44.3%;) despite Step 3 analgesic use (p < 0.001).
Fig. 1.
Pain severity by years of survivorship
Fig. 2.
Prescribed pain medication use by years of survivorship
Fig. 3.
Opioid use by years of survivorship. Using the World Health Organization (WHO) analgesia ladder: Step 0 for no analgesia, Step 1 for non-opioid pain medication, Step 2 for weak opioids, or Step 3 for strong opioids
Fig. 4.
Pain severity by opioid use. Using the World Health Organization (WHO) analgesia ladder: Step 0 for no analgesia, Step 1 for non-opioid pain medication, Step 2 for weak opioids, or Step 3 for strong opioids
Risk factors of severe pain
Table 3 shows the prevalence of severe pain by demographic, clinical, and behavioral factors. Those who received chemotherapy had higher prevalence of severe pain (10.6% treated with chemotherapy compared to 2.9% of those not reporting receiving chemotherapy; P < 0.001). We also assessed if the prevalence of severe pain varied by receipt of single modality versus multimodality treatment. We found that patients who received single modality had lower prevalence of severe pain (single = 3% versus 2 or more+ 8.6%; p = 0.045). Severe pain also varied by levels of depression as measured by CESD: 16% of those having mild to moderate risk and 22.6% among those with major risk (P < 0.001). A past diagnosis of neuropathic pain (P = 0.049) was significantly associated with severe pain at a prevalence of 14.5%, compared to 6.5% when without neuropathic pain history.
Table 3.
Characteristics of head and neck cancer survivors by severe pain
| Variable | (−) Severe Pain | (+) Severe Pain1 | P-value6 | ||
|---|---|---|---|---|---|
| N | (%) | N | (%) | ||
| Age (years) | 0.069 | ||||
| Mean ± Std.Dev | 61.8 ± 10.7 | 58.7 ± 9.6 | |||
| Sex | 0.760 | ||||
| Male | 362 | (92.8) | 28 | (7.2) | |
| Female | 103 | (92.0) | 9 | (8.0) | |
| Marital Status | 0.697 | ||||
| Single or separated | 90 | (91.8) | 8 | (8.2) | |
| Married or cohabiting | 374 | (92.8) | 29 | (7.2) | |
| Not available | |||||
| Education | 0.396 | ||||
| No college degree | 203 | (91.4) | 19 | (8.6) | |
| College graduate | 258 | (93.5) | 18 | (6.5) | |
| Not available | |||||
| Employment | 0.609 | ||||
| Not employed or retired | 237 | (93.3) | 17 | (6.7) | |
| Employed | 225 | (91.8) | 20 | (8.2) | |
| Not available | |||||
| Race | 0.768 | ||||
| Non-Hispanic White | 422 | (92.7) | 33 | (7.3) | |
| Other | 43 | (91.5) | 4 | (8.5) | |
| Survivorship (years)2 | 0.439 | ||||
| Average ± Std.Dev | 4.6 ± 4.4 | 5.2 ± 4.1 | |||
| Survivorship | 0.392 | ||||
| ≤ 1 year | 133 | (94.3) | 8 | (5.7) | |
| > 1 to ≤3 years | 105 | (92.9) | 8 | (7.1) | |
| > 3 to ≤8 years | 134 | (92.4) | 11 | (7.6) | |
| > 8 years | 93 | (90.3) | 10 | (9.7) | |
| Site of HNC | 0.092 | ||||
| Oropharynx | 245 | (91.4) | 23 | (8.6) | |
| Oral cavity | 113 | (97.4) | 3 | (2.6) | |
| Larynx | 48 | (88.9) | 6 | (11.1) | |
| Other | 59 | (92.2) | 5 | (7.8) | |
| Recurrent disease | 0.065 | ||||
| Yes | 82 | (97.6) | 2 | (2.4) | |
| No | 396 | (92.3) | 33 | (7.7) | |
| Treatment3 | |||||
| Chemotherapy | 261 | (89.4) | 31 | (10.6) | 0.001 |
| Radiotherapy | 374 | (91.7) | 34 | (8.3) | 0.089 |
| Surgery | 296 | (94.0) | 19 | (6.0) | 0.132 |
| Single modality | 129 | (97.0) | 4 | (3.0) | 0.045 |
| Multi modality | 338 | (91.4) | 32 | (8.6) | |
| Comorbidities3 | |||||
| Hypertension | 198 | (93.8) | 13 | (6.2) | 0.301 |
| Herniated disc and radiculopathy | 96 | (89.7) | 11 | (10.3) | 0.227 |
| Osteoarthritis | 55 | (87.3) | 8 | (12.7) | 0.121 |
| Neuropathic pain | 47 | (85.5) | 8 | (14.5) | 0.049 |
| Diabetes | 52 | (94.5) | 3 | (5.5) | 0.785 |
| Coronary heart disease | 43 | (91.5) | 4 | (8.5) | 0.772 |
| Emphysema and lung disease | 26 | (83.9) | 5 | (16.1) | 0.075 |
| Rheumatoid arthritis | 29 | (93.5) | 2 | (6.5) | 1.000 |
| Cerebrovascular disease | 19 | (90.5) | 2 | (9.5) | 0.671 |
| Depression Risk4 | < 0.001 | ||||
| No risk | 374 | (95.4) | 18 | (4.6) | |
| Mild-moderate risk | 63 | (84.0) | 12 | (16.0) | |
| Major risk | 24 | (77.4) | 7 | (22.6) | |
| Not available | 7 | ||||
| Analgesia Classification5 | 0.004 | ||||
| No analgesic | 335 | (95.2) | 17 | (4.8) | |
| Step 1 | 68 | (89.5) | 8 | (10.5) | |
| Step 2 | 3 | (100.0) | 0 | (0.0) | |
| Step 3 | 58 | (82.9) | 12 | (17.1) | |
| Not available | 4 | ||||
1Score of 8 to 10 according to the 2019 National Comprehensive Cancer Network guidelines
2Number of years from date of head and neck cancer diagnosis
3Categories are not mutually exclusive. Only “yes” responses are shown
4Center for Epidemiological Studies - Depression (CES-D) Scale
5World Health Organization analgesia ladder
6P value based Pearson chi-square or Student's t-test when appropriate
Table 4 shows the results of the multivariable analyses. Candidate variables included those with the p-value < 0.20 in the univariable analyses. We found the following as significant risk factors for severe pain: recurrent disease (OR 6.779, 95% CI [1.445, 31.801]), history of chemotherapy (OR 6.005, 95% CI [2.104, 17.142]), and depression (Mild-moderate OR 5.306, 95% CI [2.202, 12.783]; Major OR 8.002, 95% CI [2.672, 23.967]). When we conducted a subset analyses by including only those without recurrent disease, we found that receipt of chemotherapy and depression persisted as significant risk factors for severe pain.
Table 4.
Multivariable regression of variables associated with severe painb in survivors of head and neck cancer (n = 489)c
| Variablea | Odds ratio | 95% Confidence interval | P value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Recurrent disease | ||||
| No | 1.000 | |||
| Yes | 6.779 | 1.445 | 31.801 | 0.015 |
| Chemotherapy | ||||
| No | 1.000 | |||
| Yes | 6.005 | 2.104 | 17.142 | 0.001 |
| Depression risk | ||||
| (1) No risk | 1.000 | REF | ||
| (2) Mild-moderate risk | 5.306 | 2.202 | 12.783 | < 0.001 |
| (3) Major risk | 8.002 | 2.672 | 23.967 | < 0.001 |
aVariables entered were age (years), sex, location of malignancy, recurrent disease, chemotherapy, radiotherapy, surgery, osteoarthritis, emphysema and lung disease, neuropathic pain, and depression risk
bScore of 8 to 10 according to the 2019 National Comprehensive Cancer Network guidelines
cComplete case analysis
Discussion
The traditional concept of post-treatment surveillance in HNC patients relies on examinations directed at early detection of disease recurrence and/or second primary tumors. However, emerging evidence underscores the importance of monitoring effective management of toxicities of treatment. Consistent with previous studies [2, 5, 6], we found a high prevalence of pain in a large population of HNC survivors, with as many as 45% reporting pain during their routine clinic visit. The most frequently reported locations were in the head and neck cancer sites including the neck and throat and head and oral cavity. Pain is estimated to afflict around 20 to 40% of cancer survivors, with impacts on survival and quality of life [23–25]. Logan et al. conducted phone interviews of 5-year survivors of HNC and noted pain prevalence to be 43% [10]. A similar study by Funk et al. documented pain to occur in 30% [11]. Lower pain prevalence estimates of 25 and 26% were documented after 1 and 2 years, respectively [12]. Covering a more heterogenous group of 224 HNC survivors who were at least 15 months from treatment, Rogers et al. identified pain in 50% following examination of health-related quality of life data [13]. More recently, Cramer et al. evaluated pain in 175 HNC survivors who were ≥ 1 year after diagnosis with a median survivorship duration of 6.6 years after treatment. They found pain was reported by 45.1% [6]. In this study, we found that close to 10% of survivors who are 8 years or more from first diagnosis have severe pain, suggesting that pain remains a significant concern for long-term survivors. To our knowledge, our study is among the largest cohort study of pain in HNC survivors.
We found that only 30% of our sample reported using any pain medication, and reports of prescription medication use significantly varied by years of survivorship. Importantly, we found that the use of strong opioids (Step 3) were highest among those who are under 1 year from diagnosis. Among those with medication, we noted considerable mismatch between severity and pain medication use, with as many as 70 HNC survivors on Step 3 opioids but continuing to experience severe pain. Furthermore, as many as 120 HNC survivors who were experiencing pain did not report any pain medication, including 17 with severe pain. Indeed, the American Cancer Society guideline for HNC survivors underscored the need to recognize late and long-term complications or toxicities of cancer treatment, as well as its under-treatment and management. Managing pain in HNC survivors includes conducting a full assessment and incorporating the use of multimodal therapies [2, 16, 26]. Opioids are a mainstay of treatment for severe pain. However, chronic opioid use may result in tolerance, dependence, and hyperalgesia. Current recommendations suggest opioid rotation as well as regular evaluation and mitigation of opioid risks for abuse [16, 27]. Barriers to adequate pain management include poor pain assessment, inadequate knowledge on pain physiology and management, misconceptions regarding opioids, and a fear of addiction in light of the opioid abuse epidemic [27–29]. Personal patient decision may also factor into undertreatment of pain; new patients often use analgesics the most (as evidenced by our data), but for some, once they realize the pain is chronic they may opt out of using opioids given the side effects (impact of opioids on their quality of life, energy levels, mental agility, etc.) and turn to alternative pain therapies, including meditation, heat massage, etc. Indeed, 7% of our respondents reported use of alternative pain therapies.
In our study, we identified chemotherapy, recurrent disease, and depression as significant risk factors of severe pain. HNC is commonly treated with one or any combination of radiotherapy, chemotherapy, or surgery, all of which have certain toxicity profiles that may result in pain [2, 30, 31]. Standard treatment of HNC is based largely on primary tumor location and cancer stage. Early stage disease is treated with single modality treatment and advanced stage disease is treated with multimodal therapy [9]. As observed in previous studies, we found that patients who received multimodal therapy had a higher prevalence of severe pain [5]. In the multivariate analyses, only chemotherapy was significant in its relationship with severe pain. Surgery and radiotherapy are the primary modes of treatment in HNC, whereas chemotherapy alone does not have curative potential. However, chemotherapy has seen an increasing role in definitive treatment as an adjunct or concurrent modality for locally advanced disease [32]. Acutely, chemotherapy may contribute to the development of oral mucositis causing acute pain in this population [33]. Furthermore, chronic pain following chemotherapy may be mainly due to chemotherapy-induced peripheral neuropathy (CIPN). For example, studies show that CIPN may lead to chronic neuropathic pain, which can be difficult to control with opioids [6, 26, 34]. Furthermore, when combined with other modalities, chemotherapy has been reported to increase the risk of pain-causing events in both surgery [35] and radiotherapy [36].
Not surprisingly, a history of tumor recurrence is a significant risk factor of severe pain with up to 6.8 times higher odds in our population. The relationship of pain and recurrence is recognized in HNC and is often associated with poor prognosis. Typically, pain prompts the presence of recurrence [37–39]. However, recurrence may also require therapeutic interventions leading to treatment-related pain.
Consistent with previous studies, we found depression as a significant risk factor for pain. Past reports have noted the complex relationship between pain and depression [38, 40, 41], with depression found to be prevalent in HNC survivors who have completed treatment [38, 40, 41]. While the exact mechanism underlying pain and depression in HNC patients remains an active area of investigation, it has been proposed that cancer patients who are depressed are more susceptible to somatic discomfort, hence pain [42]. Nonetheless, our finding points to the need for early recognition of mental health needs among those with cancer and potentially the greater demand for mental health services for HNC survivors.
We note in our study that while 11.6% of HNC survivors reported a history of neuropathic pain, fewer (3.8%) indicated taking psychotropic medication such as pregabalin and gabapentin, which are typically advised for neuropathic pain [16, 43]. Pain with a neuropathic etiology does not always respond to typical analgesics including opioids [44]. However, a prior diagnosis of neuropathic pain may not necessarily be related to cancer or its treatment. Further, although the most frequently reported sites of pain were in the head and neck region, we cannot entirely attribute that it was tumor-related. Thus, neuropathic pain was considered a comorbidity in our analysis.
Among the limitations of this study is the cross-sectional survey design among HNC survivors who presented for follow-up and, therefore, may underrepresent groups that fail to consult for any reason including debilitating conditions or a general positive sense of well-being, either of which can affect pain reporting. Recall bias is also a potential limitation. In addition, although the indicated pain sites were in the head and neck region, we are unable to classify the type of pain or give some indication regarding the cause of pain; there are a number of potential sources of pain – dental disease/infection, osteoradionecrosis, soft tissue infection, tissue necrosis, neuropathic pain, muscular pain, chronic mucositis, recurrence, flap donor sites, etc. Moreover, we lack data on the type of resection or reconstruction performed during surgery. The study is also limited to a single highly specialized institution which may have a unique population, including but not limited to low representation of racial and ethnic minorities and lower income social groups. Our study also focused on survivors who have received treatment and does not include the broader survivorship population that includes newly-diagnosed patients as defined by the National Cancer Institute. It is also notable that since males are most affected by HNC [45, 46], we have a smaller number of women included in our sample. Our results, therefore, are not necessarily generalizable to broader patient populations [47, 48]. Therefore, additional studies are needed to validate our findings.
Conclusions
In conclusion, we identified a high prevalence of pain among HNC cancer survivors and determined that analgesic use varied by the duration of survivorship. Therefore, routine surveillance for pain must be consistent throughout the course of survivorship. We have identified that chemotherapy, cancer recurrence, and depression are risk factors for severe pain and may serve as prompts for thorough pain evaluation. Finally, the low utilization of pain medication among HNC survivors despite a high prevalence of pain suggests that a considerable fraction of these HNC survivors with pain are possibly undertreated. This mismatch between the high prevalence of pain and pain medication uncovers an unmet need as well as opportunities for further research and intervention to improve the management of pain among HNC survivors.
Acknowledgements
We recognize and thank the patients who participated in this study as well as Veronica Paredes, Guadalupe Padilla, Cindy Menendez, Jazmin Menendez, and Mary Lou Flores for their help in recruiting patients and data management.
Abbreviations
- HNC
Head and neck cancer
- MDACC
MD Anderson Cancer Center
- WHO
World Health Organization
- CESD
Community Epidemiologic Studies Depression Scale
- REDCap
Research Electronic Data Capture
- NCCN
National Comprehensive Cancer Network
- CIPN
Chemotherapy-induced peripheral neuropathy
Authors’ contributions
CCR is the Principal Investigator who designed the study. RDR and CRG performed the statistical analysis. CCR, RDR, and JLR drafted and substantially revised the manuscript. CCR, RDR, JLR, JDP, SJY, EYH participated in the interpretation of data. CCR, JLR, RDR, JDP, SJY, and EYH participated in reviewing and editing the manuscript. All authors read and approved the final manuscript.
Funding
National Institutes of Dental and Craniofacial Research [R01 DE022891, PI:CCR].
Availability of data and materials
The database generated in the current study are not publicly available due to ethical restrictions, but are available from corresponding author.
Declarations
Ethics approval and consent to participate
Ethical approval was approved by the local Institutional Review Board of MDACC under Protocol PA 2012–1035. All the procedures performed were part of the routine care.
Institutional Review Board of MDACC; PA 2012–1035.
Consent for publication
Institutional Review Board of MDACC; PA 2012–1035.
Competing interests
Dr. Yeung received funding for investigator-initiated clinical studies from DepMed, Inc. and Bristol-Myer-Squibb. The other authors certify that they have no affiliation with or involvement in any organization or entity with any financial interest in the subject matter discussed in this manuscript.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jenny L. Ren and Raniv D. Rojo contributed equally to this work.
Contributor Information
Jenny L. Ren, Email: Jenny.Ren@bcm.edu
Raniv D. Rojo, Email: raniv.rojo@up.edu.ph
Joy Vanessa D. Perez, Email: jdperez@mdanderson.org
Sai-Ching J. Yeung, Email: syeung@mdanderson.org
Ehab Y. Hanna, Email: eyhanna@mdanderson.org
Cielito C. Reyes-Gibby, Email: creyes@mdanderson.org
References
- 1.National Cancer Institute - Division of Cancer Control & Population Sciences . Survivorship definitions. 2019. [Google Scholar]
- 2.Ing JW. Head and neck cancer pain. Otolaryngol Clin N Am. 2017;50(4):793–806. doi: 10.1016/j.otc.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Brandenbarg D, Maass SWMC, Geerse OP, Stegmann ME, Handberg C, Schroevers MJ, et al. A systematic review on the prevalence of symptoms of depression, anxiety and distress in long-term cancer survivors: implications for primary care. Eur J Cancer Care (Engl) 2019;28(3):e13086. doi: 10.1111/ecc.13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Götze H, Friedrich M, Taubenheim S, Dietz A, Lordick F, Mehnert A. Depression and anxiety in long-term survivors 5 and 10 years after cancer diagnosis. Support Care Cancer. 2020;28(1):211–220. doi: 10.1007/s00520-019-04805-1. [DOI] [PubMed] [Google Scholar]
- 5.Bossi P, Giusti R, Tarsitano A, Airoldi M, De Sanctis V, Caspiani O, et al. The point of pain in head and neck cancer. Crit Rev Oncol Hematol. 2019;138:51–59. doi: 10.1016/j.critrevonc.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Cramer JD, Johnson JT, Nilsen ML. Pain in head and neck cancer survivors: prevalence, predictors, and quality-of-life impact. Otolaryngol Head Neck Surg. 2018;159(5):853–858. doi: 10.1177/0194599818783964. [DOI] [PubMed] [Google Scholar]
- 7.Treanor C, Donnelly M. Late effects of cancer and cancer treatment--the perspective of the patient. Support Care Cancer. 2016;24 Available from: https://pubmed.ncbi.nlm.nih.gov/26066051/?from_filter=ds1.y_5&from_linkname=pubmed_pubmed_citedin&from_from_uid=20848873&from_page=9&from_pos=6. Cited 2020 May 20. [DOI] [PubMed]
- 8.National Institutes of Health NIH state-of-the-science statement on symptom management in cancer: pain, depression, and fatigue. NIH Consens State Sci Statements. 2002;19(4):1–29. [PubMed] [Google Scholar]
- 9.Cohen EEW, LaMonte SJ, Erb NL, Beckman KL, Sadeghi N, Hutcheson KA, et al. American Cancer society head and neck cancer survivorship care guideline. CA Cancer J Clin. 2016;66(3):203–239. doi: 10.3322/caac.21343. [DOI] [PubMed] [Google Scholar]
- 10.Logan HL, Bartoshuk LM, Fillingim RB, Tomar SL, Mendenhall WM. Metallic taste phantom predicts oral pain among 5-year survivors of head and neck cancer. Pain. 2008;140(2):323–331. doi: 10.1016/j.pain.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Funk GF, Karnell LH, Christensen AJ. Long-term health-related quality of life in survivors of head and neck cancer. Arch Otolaryngol Head Neck Surg. 2012;138(2):123–133. doi: 10.1001/archoto.2011.234. [DOI] [PubMed] [Google Scholar]
- 12.Chaplin JM, Morton RP. A prospective, longitudinal study of pain in head and neck cancer patients. Head Neck. 1999;21(6):531–537. doi: 10.1002/(sici)1097-0347(199909)21:6<531::aid-hed6>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 13.Rogers SN, O’Donnell JP, Williams-Hewitt S, Christensen JC, Lowe D. Health-related quality of life measured by the UW-QoL—reference values from a general dental practice. Oral Oncol. 2006;42(3):281–287. doi: 10.1016/j.oraloncology.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Bennett MI, Smith BH, Torrance N, Potter J. The S-LANSS score for identifying pain of predominantly neuropathic origin: validation for use in clinical and postal research. J Pain. 2005;6(3):149–158. doi: 10.1016/j.jpain.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Oldenmenger WH, de Raaf PJ, de Klerk C, van Der Rijt CC. Cut points on 0-10 numeric rating scales for symptoms included in the Edmonton symptom assessment scale in cancer patients: a systematic review. J Pain Symptom Manag. 2013;45(6):1083–1093. doi: 10.1016/j.jpainsymman.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Swarm RA, Paice JA, Anghelescu DL, Are M, Bruce JY, Buga S, et al. Adult cancer pain, version 3.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2019;17(8):977–1007. doi: 10.6004/jnccn.2019.0038. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization . Cancer pain relief. Geneva: World Health Organization; 1986. [Google Scholar]
- 18.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 19.Geisser ME, Roth RS, Robinson ME. Assessing depression among persons with chronic pain using the Center for Epidemiological Studies-Depression Scale and the Beck Depression Inventory: a comparative analysis. Clin J Pain. 1997;13(2):163–170. doi: 10.1097/00002508-199706000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bendel RB, Afifi AA. Comparison of stopping rules in forward “stepwise” regression. J Am Stat Assoc. 1977;72(357):46–53. [Google Scholar]
- 22.Cohen N, Fedewa S, Chen AY. Epidemiology and demographics of the head and neck cancer population. Oral Maxillofac Surg Clin N Am. 2018;30(4):381–395. doi: 10.1016/j.coms.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 23.van den Beuken-van Everdingen MHJ, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 2007;18(9):1437–1449. doi: 10.1093/annonc/mdm056. [DOI] [PubMed] [Google Scholar]
- 24.Jiang C, Wang H, Wang Q, Luo Y, Sidlow R, Han X. Prevalence of chronic pain and high-impact chronic pain in cancer survivors in the United States. JAMA Oncol. 2019;5(8):1224–1226. doi: 10.1001/jamaoncol.2019.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanford NN, Sher DJ, Butler SS, Xu X, Ahn C, Aizer AA, et al. Prevalence of chronic pain among cancer survivors in the United States, 2010-2017. Cancer. 2019;125(23):4310–4318. doi: 10.1002/cncr.32450. [DOI] [PubMed] [Google Scholar]
- 26.Paice JA, Portenoy R, Lacchetti C, Campbell T, Cheville A, Citron M, et al. Management of chronic pain in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2016;34(27):3325–3345. doi: 10.1200/JCO.2016.68.5206. [DOI] [PubMed] [Google Scholar]
- 27.Paice JA. Pain in cancer survivors: how to manage. Curr Treat Options in Oncol. 2019;20(6):48. doi: 10.1007/s11864-019-0647-0. [DOI] [PubMed] [Google Scholar]
- 28.Cluxton C. The challenge of cancer pain assessment. Ulster Med J. 2019;88(1):43–46. [PMC free article] [PubMed] [Google Scholar]
- 29.Paice JA. Managing pain in patients and survivors: challenges within the United States opioid crisis. J Natl Compr Cancer Netw. 2019;17(5.5):595–598. doi: 10.6004/jnccn.2019.5010. [DOI] [PubMed] [Google Scholar]
- 30.Chen S-CC, Liao C-TT, Chang JT-CC. Orofacial pain and predictors in oral squamous cell carcinoma patients receiving treatment. Oral Oncol. 2011;47(2):131–135. doi: 10.1016/j.oraloncology.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Bossi P, Ghiani M, Argenone A, Depenni R. Is pain part of a systemic syndrome in head and neck cancer? Support Care Cancer. 2020;28(2):451–459. doi: 10.1007/s00520-019-05147-8. [DOI] [PubMed] [Google Scholar]
- 32.Sindhu SK, Bauman JE. Current concepts in chemotherapy for head and neck cancer. Oral Maxillofac Surg Clin N Am. 2019;31(1):145–154. doi: 10.1016/j.coms.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lalla RV, Brennan MT, Gordon SM, Sonis ST, Rosenthal DI, Keefe DM. Oral mucositis due to high-dose chemotherapy and/or head and neck radiation therapy. J Natl Cancer Inst Monogr. 2019;2019(53):lgz011. doi: 10.1093/jncimonographs/lgz011. [DOI] [PubMed] [Google Scholar]
- 34.Epstein JB, Miaskowski C. Oral pain in the cancer patient. J Natl Cancer Inst Monogr. 2019;2019(53):lgz003. doi: 10.1093/jncimonographs/lgz003. [DOI] [PubMed] [Google Scholar]
- 35.Burton AW, Fanciullo GJ, Beasley RD, Fisch MJ. Chronic pain in the cancer survivor: a new frontier. Pain Med. 2007;8(2):189–198. doi: 10.1111/j.1526-4637.2006.00220.x. [DOI] [PubMed] [Google Scholar]
- 36.Mirabile A, Airoldi M, Ripamonti C, Bolner A, Murphy B, Russi E, et al. Pain management in head and neck cancer patients undergoing chemo-radiotherapy: clinical practical recommendations. Crit Rev Oncol Hematol. 2016;99:100–106. doi: 10.1016/j.critrevonc.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 37.Smit M, Balm AJ, Hilgers FJ, Tan IB. Pain as sign of recurrent disease in head and neck squamous cell carcinoma. Head Neck. 2001;23(5):372–375. doi: 10.1002/hed.1046. [DOI] [PubMed] [Google Scholar]
- 38.Scharpf J, Karnell LH, Christensen AJ, Funk GF. The role of pain in head and neck cancer recurrence and survivorship. Arch Otolaryngol Neck Surg. 2009;135(8):789. doi: 10.1001/archoto.2009.107. [DOI] [PubMed] [Google Scholar]
- 39.Srivastava P, Kingsley PA, Srivastava H, Sachdeva J, Kaur P. Persistent post-radiotherapy pain and locoregional recurrence in head and neck cncer-is there a hidden link? Korean J Pain. 2015;28(2):116–121. doi: 10.3344/kjp.2015.28.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haisfield-Wolfe ME, McGuire DB, Soeken K, Geiger-Brown J, De Forge BR. Prevalence and correlates of depression among patients with head and neck cancer: a systematic review of implications for research. Oncol Nurs Forum. 2009;36(3):E107–E125. doi: 10.1188/09.ONF.E107-E125. [DOI] [PubMed] [Google Scholar]
- 41.Chen AM, Daly ME, Vazquez E, Courquin J, Luu Q, Donald PJ, et al. Depression among long-term survivors of head and neck cancer treated with radiation therapy. JAMA Otolaryngol Neck Surg. 2013;139(9):885. doi: 10.1001/jamaoto.2013.4072. [DOI] [PubMed] [Google Scholar]
- 42.Tesch RS, Denardin OVP, Baptista CA, Dias FL. Depression levels in chronic orofacial pain patients: a pilot study. J Oral Rehabil. 2004;31(10):926–932. doi: 10.1111/j.1365-2842.2004.01379.x. [DOI] [PubMed] [Google Scholar]
- 43.McMenamin EM, Grant M. Pain prevention using head and neck cancer as a model. J Adv Pract Oncol. 2015;6(1):44–49. [PMC free article] [PubMed] [Google Scholar]
- 44.Edwards HL, Mulvey MR, Bennett MI. Cancer-related neuropathic pain. Cancers. 2019;11(3) Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6468770/. Cited 2020 Nov 16. [DOI] [PMC free article] [PubMed]
- 45.Mourad M, Jetmore T, Jategaonkar AA, Moubayed S, Moshier E, Urken ML. Epidemiological trends of head and neck cancer in the United States: a SEER population study. J Oral Maxillofac Surg. 2017;75(12):2562–2572. doi: 10.1016/j.joms.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 47.Fakhry C, Westra WH, Wang SJ, van Zante A, Zhang Y, Rettig E, et al. The prognostic role of sex, race, and human papillomavirus in oropharyngeal and nonoropharyngeal head and neck squamous cell cancer. Cancer. 2017;123(9):1566–1575. doi: 10.1002/cncr.30353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson-Harvey A, Yetukuri M, Hansen AR, Simpson MC, Adjei Boakye E, Varvares MA, et al. Rising incidence of late-stage head and neck cancer in the United States. Cancer. 2020;126(5):1090–1101. doi: 10.1002/cncr.32583. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The database generated in the current study are not publicly available due to ethical restrictions, but are available from corresponding author.