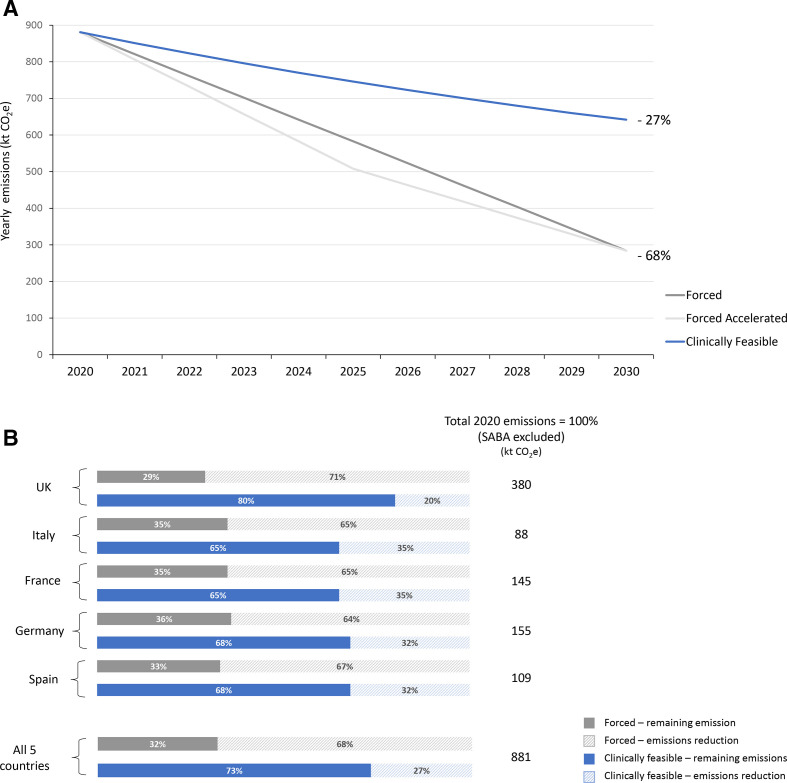
Figure 1.
Total CO2e emissions reduction, by 2030, in the scenario of switch from pMDIs to DPI/SMIs (excluding SABA inhalers) in the three subscenarios of switch (A) % reduction trends across the five countries and (B) % reduction for the five countries + total in the case of switch to DPI/SMIs with forced or clinically feasible subscenarios (for single countries UK, Italy, France, Germany and Spain, see online supplemental figure 1). Both forced subscenario strategies result in greater yearly emissions reductions than the clinically feasible scenario across the 10-year assessment period for each reference market. Forced: substitution of 80% of pMDIs with DPI/SMIs by 2030, assuming a linear trend; forced accelerated: substitution of 80% of pMDIs with DPI/SMIs by 2030, but with 50% substitution by 2025 and the remaining 30% achieved between 2025 and 2030; clinically feasible: based on historical data, every year there is substitution of pMDIs with DPI/SMIs for 5% of the previous year’s pMDI sales, resulting in a non-linear trend and a final reduction of 40% by 2030. SABA inhalers were excluded in this scenario. CO2e, carbon dioxide equivalent; DPI, dry-powder inhaler; kt, kilo tons; pMDI, pressurised metered-dose inhaler; SABA, short-acting β2-agonist; SMI, soft mist inhaler.