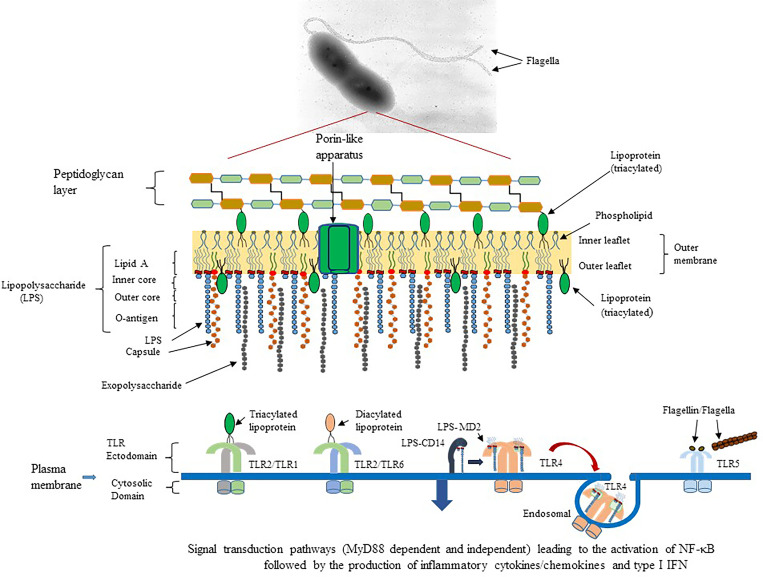
Figure 1.
Schematic diagram of potential outer membrane components of Gram-negative pathogens that could interact with surface expressed TLR of the host’s innate immune system. An example of B. pseudomallei 1026b, a motile, human pathogen that causes melioidosis, and the presence of pathogen associated molecular patterns (PAMPs) or molecules that can activate TLR of the host is presented (Kawai and Akira, 2010). TLR2/TLR1 (heterodimer) could be activated by triacylated lipoproteins that are embedded in the inner and outer leaflet of the bacterial membrane. TLR2/TLR6 (heterodimer) can be activated by diacylated lipoproteins that are usually found in Gram-positive bacteria. TLR4 (homodimer) is activated by lipid A which is part of the LPS moiety found in the outer membrane of Gram-negative bacteria. LPS molecules are recognized by CD14 and transferred to the MD2-TLR4 complex before activation of TLR4. A subset of the LPS-MD-TLR4 complex is brought into the host cell by endocytosis, which is facilitated by CD14. TLR5 (homodimer) is activated by the flagellin molecule, which is the subunit of flagella. Activation requires at least one flagellin molecule per TLR5 molecule for optimal activation of the homodimer. In addition, TLR5 can be activated by flagella, which we show in the current report. All surfaced expressed TLRs transmit their activation signal across the host membrane into the cytoplasm and recruit the signal transduction protein MyD88 and TIRAP to transmit the signal leading to activation of NF-κB and expression of AP-1 to stimulate proinflammatory cytokine/chemokine production. However, the endosomal LPS-MD2-TLR4 complex recruits TRIF and TRAM to transmit the signal for TLR4 activation, which leads to the activation of NF-κB and induction of proinflammatory cytokines/chemokines and antimicrobial type I IFNs.