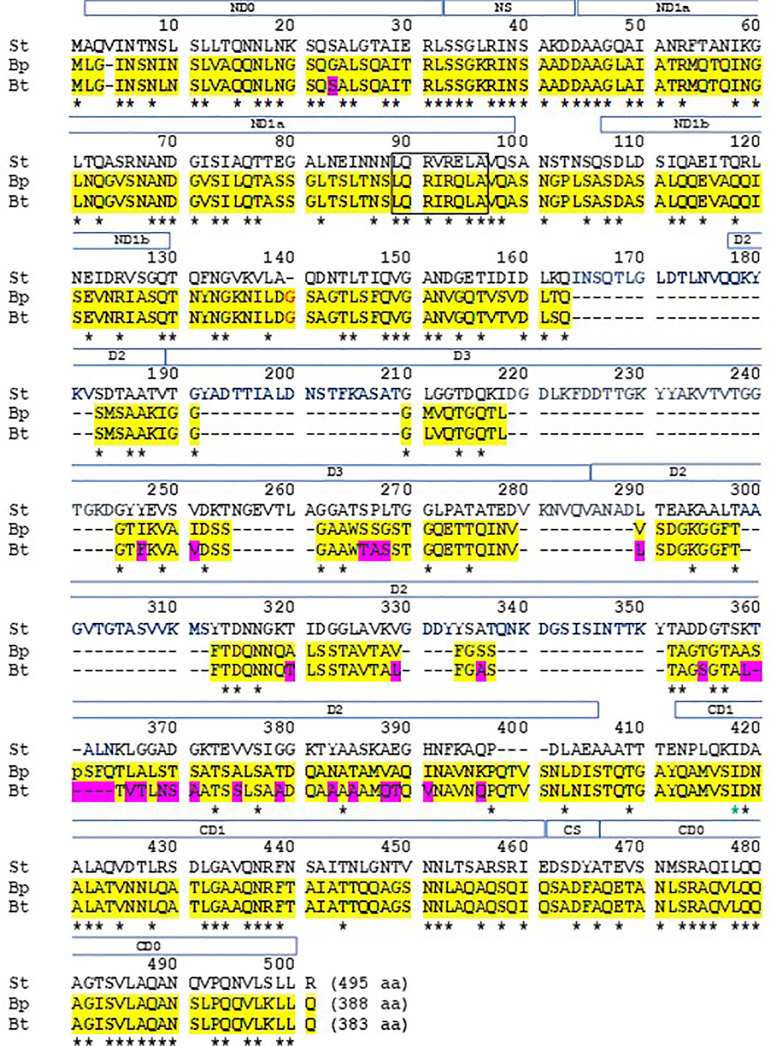
Figure 9.
Alignment of amino acid sequences of St LT2, Bp K96243, and Bt E264 FliC reveals common sequences required for folding and TLR5 activation. The amino acid sequences of FliC (495 aa, 388 aa, and 383 aa, respectively) from the three microorganisms were aligned for optimal identity (Papadopoulos and Agarwala, 2007). The amino acid sequences of FliC of Bp K96243 and Bt E264 are highlighted in yellow with differences between the two sequences highlighted in red. The asterisk below the three sequences represent identical amino acids for all three FliCs. The dashes are spaces between amino acids for missing amino acids in the three FliCs. The amino acids within the square (89−96 aa) have been reported to be required for recognition by TLR5 (Smith et al., 2003; Andersen-Nissen et al., 2005). The open boxed region above the sequences represent structural domains (D0, D1, D2, and D3) of FliC that are involved in tertiary formation of the molecule and are found in both N- and C-terminal regions (regions preceded by N or C, respectively) of the molecule. (Smith et al., 2003; Yonekura et al., 2003; Andersen-Nissen et al., 2005; Yoon et al., 2012). Within the domains there are subregions, and between D0 and D1 there is a small “spoke” (S) region at both N-terminal (NS) and C-terminal regions (CS) (Smith et al., 2003; Yonekura et al., 2003; Andersen-Nissen et al., 2007; Yoon et al., 2012).