FIGURE 3. RYK family WIF domain structure and comparison with WIF-1.
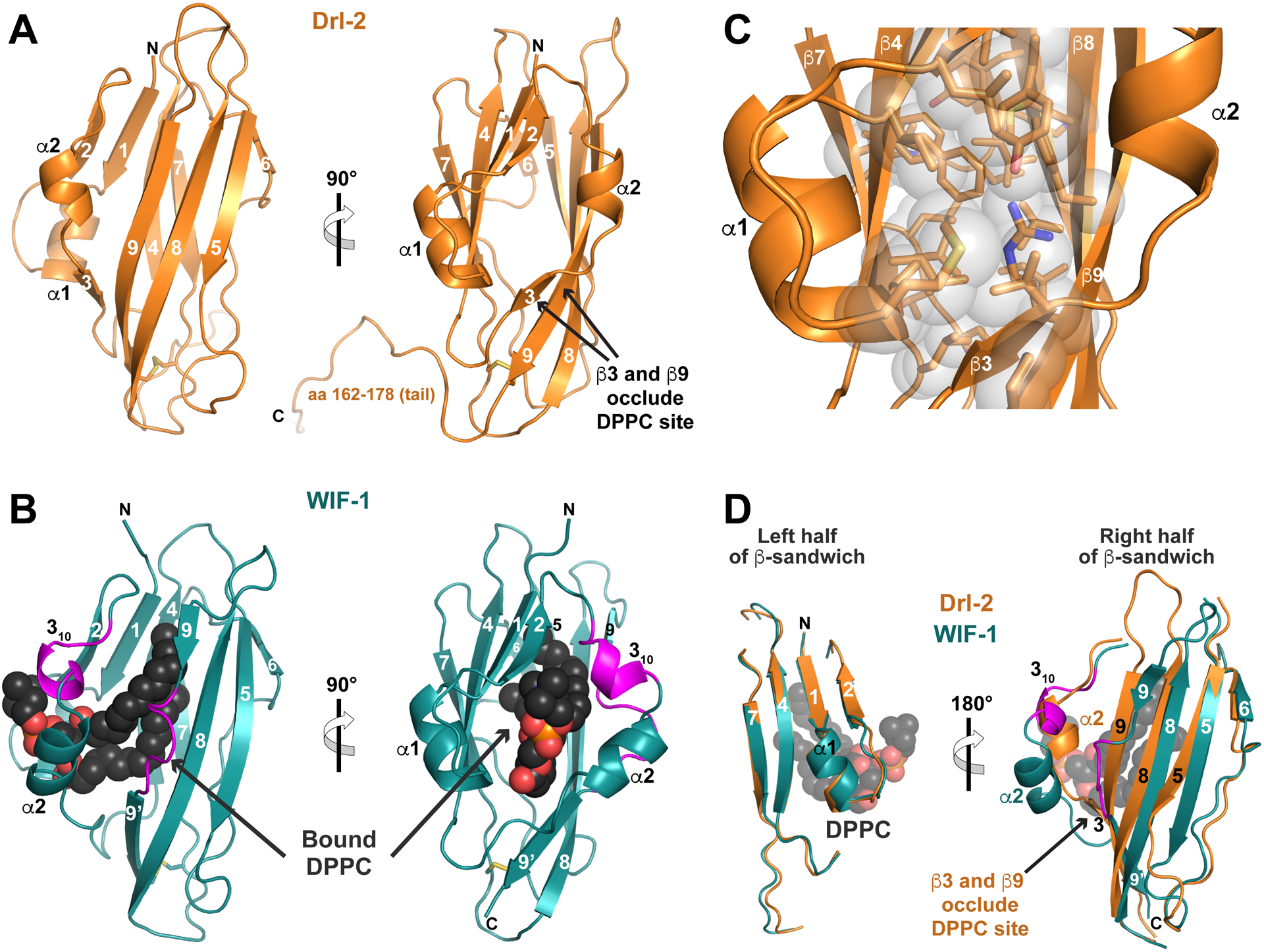
(A) Structure of the complete Drl-2 ECR (colored orange) in cartoon representation, shown in two orthogonal views. Secondary structure elements are marked – using the designation introduced by Liepinsh et al. (2006). The WIF domain ends around residue 161 (see Figure S3), and the remainder of the ECR (aa 162–182) is involved in crystal packing.
(B) Cartoon view of the WIF domain from human WIF-1 (PDBID: 2YGN), overlaid and shown in the same orientations as in A (Malinauskas et al., 2011). The WIF domain is colored deep teal, and the bound DPPC molecule is shown as black spheres (red and orange for phosphates). The two regions described as inserts in the WIF-1 WIF domain (a 310 helix and insert in β9) compared with that in Drl-2 are colored magenta.
(C) Closer view of the hydrophobic core of the Drl-2 WIF domain, using the same orientation as the right-hand side of A, showing that it is well packed, with no cavity capable of accommodating a lipid molecule.
(D) Overlay of the Drl-2 and WIF-1 WIF domains in two halves as described in the text. The half of the sandwich including β1, β2, β4, β7, and α1 overlays very well. The other half shows more deviations, particularly around α2 (where the magenta insert forms a 310 helix) and in β9 (where a magenta loop/bulge is seen). These changes allow DPPC to bind WIF-1’s WIF domain, whereas the longer (and straight) β9 – along with β3 – occludes the potential lipid-binding site in Drl-2 (and likely other RYK family members).
See also Figure S3.
