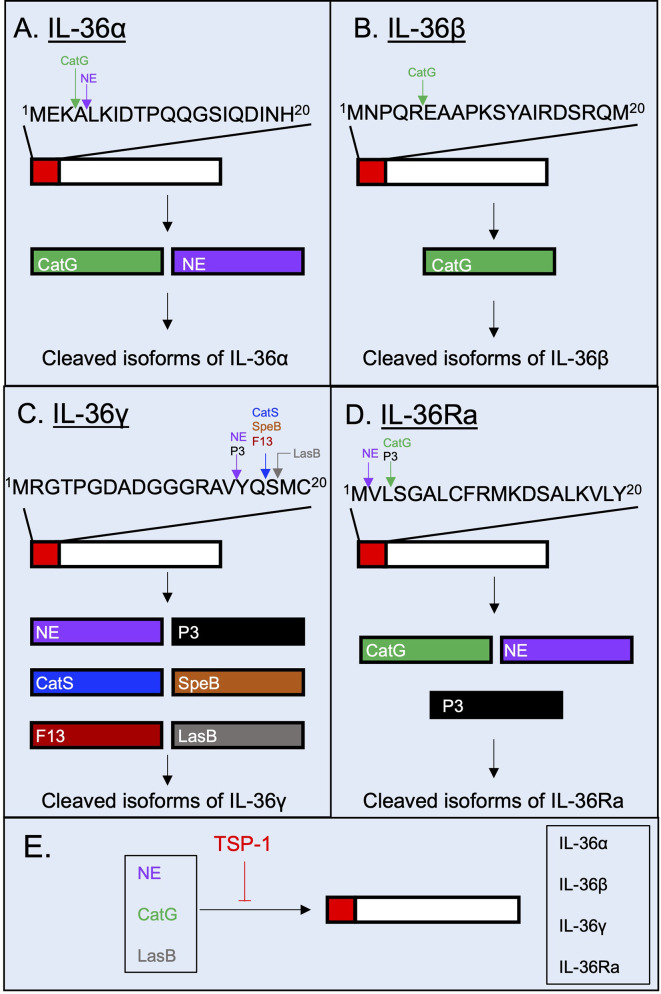
Figure 3.
Post-translational processing of human IL-36 cytokines by extracellular proteases. Human IL-36 cytokines are selectively cleaved and potentially activated by proteases. (A) IL-36α is cleaved extracellularly by neutrophil proteases Cathepsin G (CatG) and Neutrophil Elastase (NE). (B) IL-36β has been described to be N-terminally processed by CatG. (C) IL-36γ is selectively cleaved by Proteinase-3 (P3), NE, Cathepsin S (CatS), the Streptococcus pyogenes-derived protease SpeB, the Aspergillus fumigatus-derived protease F13 and the Pseudomonas aeruginosa-derived protease LasB. (D) IL-36Ra is cleaved and potentially activated by NE, CatG and P3. (E) The proteolytic cleavage of IL-36 cytokines can be regulated by the host glycoprotein thrombospondin-1, which has been shown to possess inhibitory properties against the host proteases NE and CatG, and against the bacterial protease LasB.