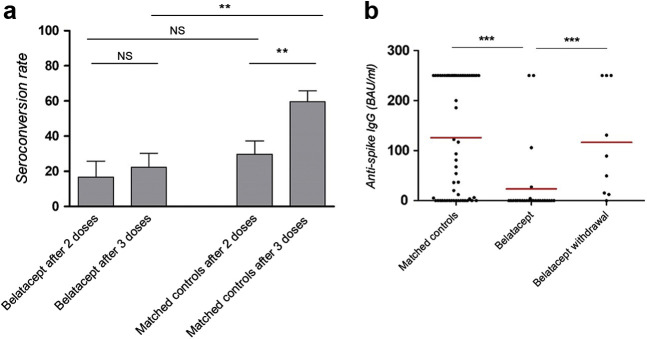
Figure 2.
Serologic assessment was performed by ECLIA Roche, Architect Abbott, or Diasorin technologies, and anti-spike IgG titers were expressed in binding antibody unit (BAU)/ml. Positivity was set as anti-spike IgG superior to laboratory threshold. Median times of serologic screening in the belatacept group and the matched control group were 42 and 36 days, respectively, after the third injection. (a) Seroconversion rate after 2 and 3 mRNA injections (i.e., anti-spike IgG level superior to laboratory threshold) in patients receiving belatacept and matched controls. (b) Anti-spike IgGs, expressed in BAU/ml, and their respective mean titer 1 month after the last vaccine injection in belatacept recipients and matched controls. Anti-spike IgGs, expressed in BAU/ml, and their respective mean titer 1 month after the last vaccine injection in belatacept recipients and patients who underwent belatacept withdrawal. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. NS, nonsignificant difference.