Fig. 1. Development of ideas related to the conception of the OXPHOS system organization in mitochondria, from the initial “solid” model to the proposed subcompartmented oxphosomic one.
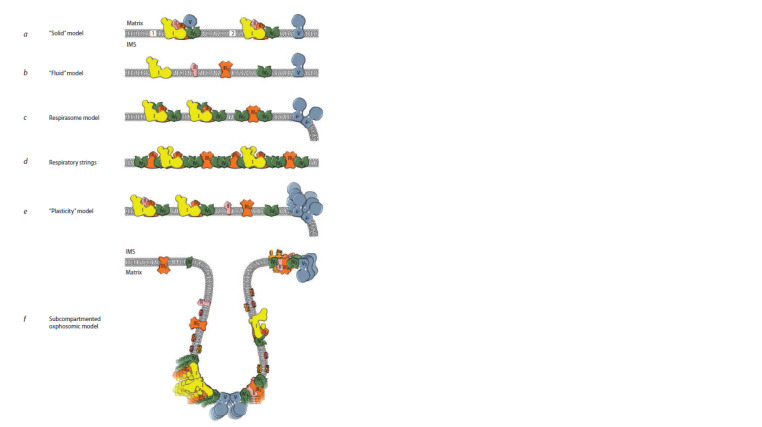
The interpretation of the models is presented taking into account recent data on the structure of OXPHOS complexes of mammals (a–e) and plants (f ). According to the literary data, all the principal models (with the exception of model f ) are given for mammalian mitochondria. Denotations: (1) initial and (2) later “solid” models; matrix and IMS – matrix and, respectively, intermembrane space of mitochondria; complexes I, II, III2, IV and IV2 of the respiratory chain and ATP-synthase (complex V) are shown in yellow, pink, orange, green and blue, respectively. It is shown on more recent schemes that ATP-synthase dimers bend the membrane and, thereby, are involved in the formation of cristae (c, e, f ). Description of the models can be found in the text. The respirasome model (c) is given in accordance with the schemes of H. Schägger (Schägger, Pfeiffer, 2000; Schägger, 2002); it ref lects the principal postulate of the model, which determines the ratio of the large supercomplex I1III2IV4 and the small supercomplex III2IV4 as 2:1. The model of respiratory strings (d) is shown according to the scheme of H. Schägger and I. Wittig (Wittig, Schägger, 2009), but, unlike the representation in the original, the f igure shows a side view (in the plane of the membrane). There are other variants of the respiratory strings (Bultema et al., 2009; Miranda-Astudillo et al., 2018). In the oxphosomic model (f ), developed for the mitochondrial OXPHOS system of etiolated pea shoots (Ukolova et al., 2020), in addition to the main OXPHOS complexes, there are also freely located alternative enzymes, which indicates a more complex organization of the phosphorylating system in plants.
