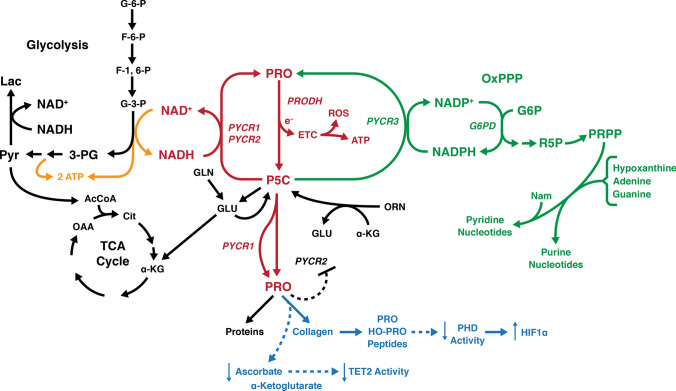
Fig. 2.
Current regulatory model for “Proline Metabolism and Cancer Reprogramming.” The “Proline-P5C Cycle” is shown in red with proline dehydrogenase transferring electrons from proline to the ETC in mitochondria which can generate ATP or ROS. Shown in green is the interlock between PYCR3 and glucose-6-P Dehydrogenase coupled by NADP+/NADPH in the cytosol. This linkage allows P5C to increase the production of PRPP and the generation or maintenance of pyridine and purine nucleotides. Shown in orange, the coupling of PYCR1/2 to the glycolytic pathway and the production of collagen shown in blue. The regeneration of NAD+ increases the flux through the glycolytic pathway, obviating the diversion of pyruvate to lactate. Instead, pyruvate is converted to acetyl CoA into the TCA Cycle. The increased synthesis of collagen from biosynthesized proline consumes acorbate and α-ketoglutarate, two substrates necessary for TET2 activity. The degradation of collagen generates prolyl and hydroxyprolyl peptides which are inhibitors of PHDs, aka EglNs, activity which will increase the levels of HIFs. Other regulatory links between PYCR1 and extracellular matrix exist. Their identity and mechanisms have been reviewed (D’Aniello et al. 2020). For intracellular localization of the respective enzymes (Phang 2019)