FIGURE 2.
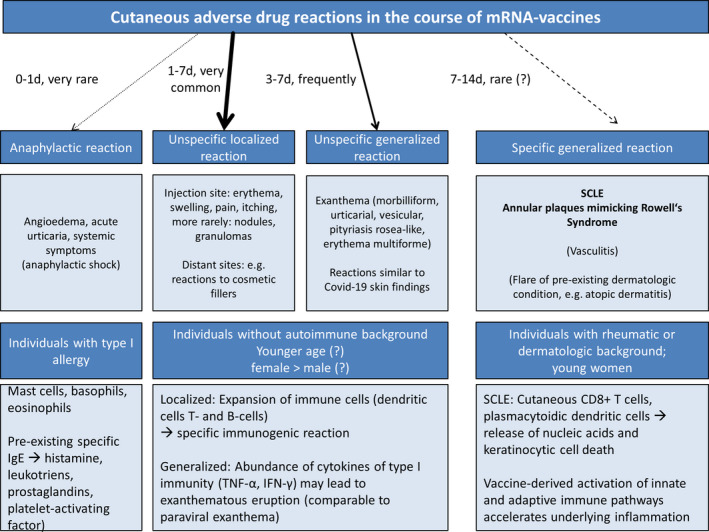
Scheme of expected cutaneous adverse drug reactions (ADR) depending on different patient groups. The vast majority of cutaneous ADR comprise unspecific localized or generalized reactions which typically occur between 1 and 7 days after the first dose; reactions after the second dose tend to occur earlier. Type I allergic reactions are very rare and occur in previously sensitized individuals (pre‐existing specific immunoglobulin E against components of the vaccine). We expect to see more specific cutaneous adverse reactions in susceptible individuals with rheumatic or dermatological background, including cutaneous lupus erythematosus, as these patients were underrepresented in clinical trials and data remains scarce. Young women are most often affected by autoimmune diseases and have not been vaccinated on a large scale with health‐care workers as an exemption.
