Figure 2. Participants in Pivotal Trials Compared With Ethnicity Breakdown of the US Population by Yeara.
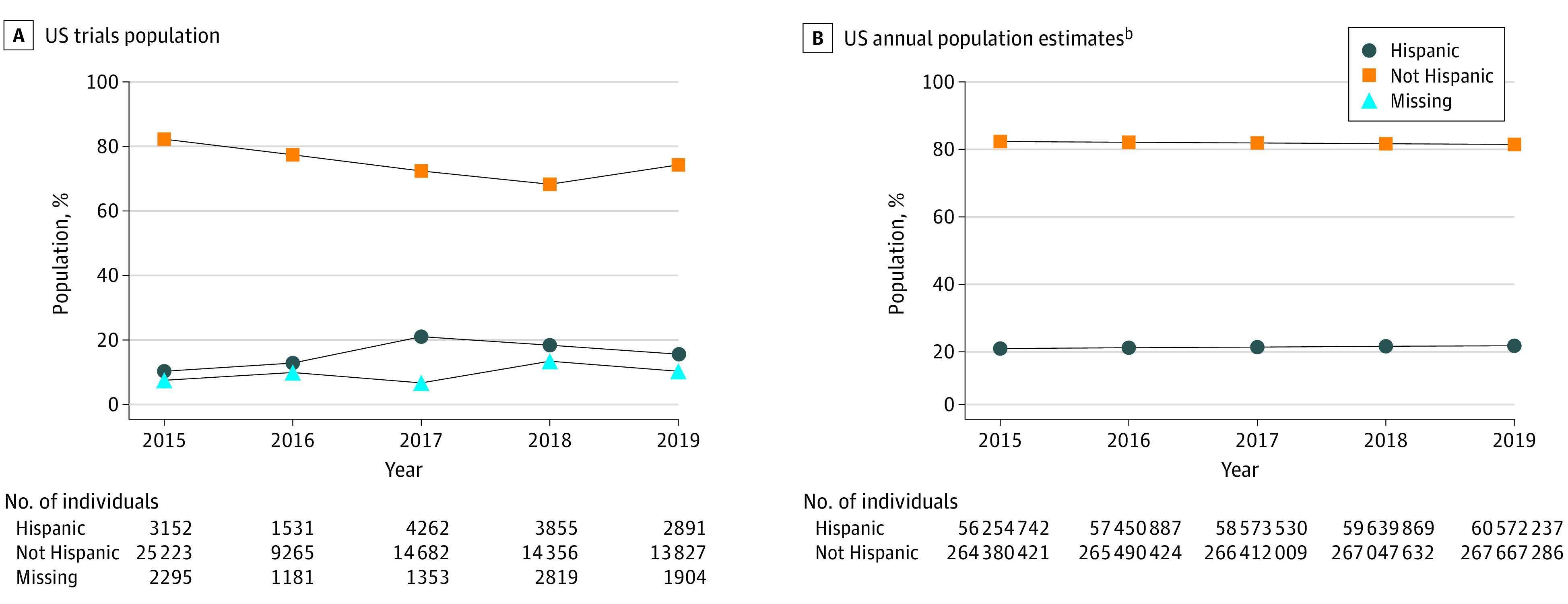
aPer the US Food and Drug Administration (FDA) report Collection, Analysis, and Availability of Demographic Subgroup Data for FDA-Approved Medical Products, August 2013, the FDA defines pivotal clinical trials as clinical investigations designed to collect definitive evidence of the safety and effectiveness of a medical product for a specified intended use (https://www.fda.gov/files/about%20fda/published/Collection–Analysis–and-Availability-of-Demographic-Subgroup-Data-for-FDA-Approved-Medical-Products.pdf).
bThere were no values for missing ethnicity data in US Census estimates.
