Abstract
We describe a case of a 20‐year‐old healthy man developing chest pain and classical symptoms of vaccine reactogenicity 12 h after receiving the first dose of mRNA‐1273 (Moderna). Cardiac troponin T was increased, and subepicardial inflammation and focal contractile dysfunction were detected by cardiac magnetic resonance imaging and echocardiography. We confirmed the diagnosis of acute myocarditis by endomyocardial biopsy demonstrating significant infiltration of monocytes and T lymphocytes. Although we detected IgG against nucleocapsid protein of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) indicating prior infection, the patient repeatedly tested negative for SARS‐CoV‐2 and had been asymptomatic for several months. Furthermore, viral genome analysis of endomyocardial biopsy samples was negative for SARS‐CoV‐2 and other potential cardiotropic viruses. These findings and the strong temporal relation between the vaccination and the symptom onset imply a potential side effect of mRNA‐1273.
Keywords: COVID‐19, Vaccine, Myocarditis, mRNA‐1273
Introduction
Coronavirus disease 2019 (COVID‐19) caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has been associated with cardiovascular complications such as thrombo‐embolism, acute coronary syndrome, arrhythmia, and myocarditis. 1 , 2 Interestingly, similar adverse events have also been observed in relation to COVID‐19 vaccination. For example, venous sinus thrombosis and thrombocytopenia may occur in recipients of the ChAdOx1 nCoV‐19 vaccine from Oxford–AstraZeneca. 3 Recently, cases of suspected myocarditis in people receiving the mRNA vaccines Comirnaty (BioNTech/Pfizer) or mRNA‐1273 (Moderna) have been reported in Israel and in the USA. 4 , 5 , 6 , 7 In Europe, the European Medicines Agency also stated that it was assessing reports of myocarditis associated with Comirnaty and mRNA‐1273. 8
Because of the manifold aetiology of myocarditis and its difficult diagnosis, extensive investigations will probably be necessary to clarify this issue. Herein, we present a case of myocarditis developing rapidly after the first dose of mRNA‐1273 in a patient with former asymptomatic SARS‐CoV‐2 infection. In contrast to previous reports, we were able to verify and characterize myocarditis by endomyocardial biopsy (EMB). Furthermore, we conducted analyses to demonstrate that alternative mechanisms are unlikely.
Case report
A 20‐year‐old male patient was transferred to our hospital because of persistent chest pain. He received his first dose of COVID‐19 vaccine Moderna 3 days ago. The next morning, about 12 h after the injection, the patient woke up with shivering, fever over 39°C, myalgia, fatigue, and growing mid‐sternal burning chest pain without radiation. Although the flu‐like symptoms vanished after 1 day, the chest pain intensified rendering him to seek emergency care.
The patient was an otherwise healthy man (height: 172 cm; weight: 69 kg; and body mass index: 23.3 kg/m2), who had no family history of cardiovascular diseases. He was on no medication. He did not take part in competitive sport and had a moderate electronic cigarette use. He denied recreational drug use, allergies, exposure to chemicals, recent travel or sick contacts, or previous adverse reactions to vaccines. Notably, he reported not having suffered any respiratory infection or enteritis for the last 6 months.
The physical examination and the chest X‐ray were normal. The resting electrocardiogram was also normal except for features of pre‐excitation (short PR interval, delta waves, and discordant T waves). Holter monitoring showed no relevant arrythmias. Blood tests revealed a 20‐fold increase in high‐sensitivity troponin T (333 pg/mL). Creatine kinase (6.89 µmol/l*s), N‐terminal pro‐B‐type natriuretic peptide (330 pg/mL), and C‐reactive protein (19.6 mg/L) were slightly elevated. A complete blood count with differential showed a marginal thrombocytopenia (119 Gpt/L) and normal eosinophil count. Cardiac troponin T rapidly diminished after 12 h and was normalized after 7 days.
Based on the medical history and clinical presentation of the patient, we suspected myocarditis and performed cardiac magnetic resonance imaging. We found subepicardial and intramural elevations in native T1 and T2 as well as marked late gadolinium enhancement primarily in the mid and basal inferolateral segments, which indicates myocardial injury and oedema in these sites. Although left ventricular ejection fraction was preserved (53–56%) as measured by magnetic resonance imaging and echocardiography, speckle tracking analysis exposed a considerable reduction in longitudinal strain down to −15% in the affected segments (Figure 1 ).
Figure 1.
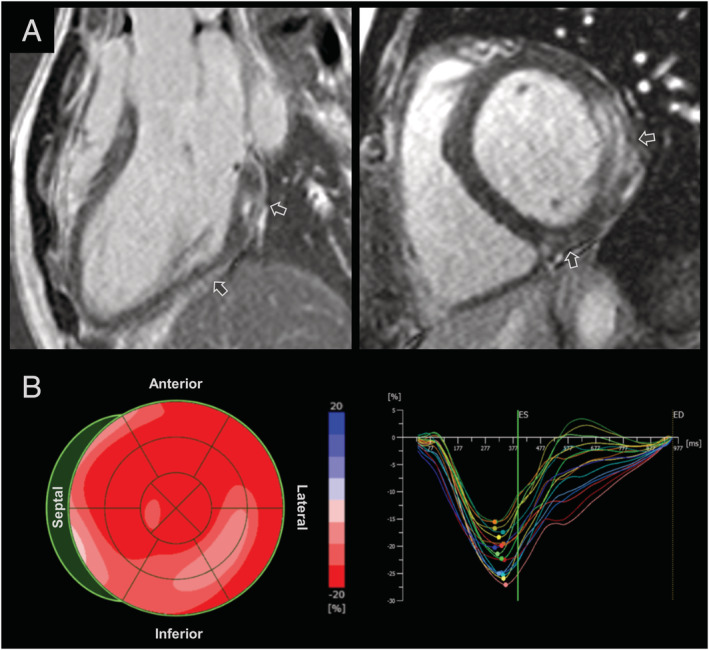
Cardiac magnetic resonance imaging and speckle tracking echocardiography. (A) Subepicardial and intramural late gadolinium enhancement (arrows) in mid and basal inferolateral segments. (B) Reduced longitudinal strains in mid and basal inferolateral as well as basal septal segments.
The patient repeatedly tested negative for SARS‐CoV‐2 by quantitative PCR of nasopharyngeal swabs. Serological assays 9 days after the vaccination detected high titres of IgG (>400 AU/mL) and IgM against SARS‐CoV‐2. However, further analysis by immunoblotting identified not only IgG against the spike protein of SARS‐CoV‐2 but also IgG against its nucleocapsid protein, which indicates prior infection. We also found slightly elevated levels (two‐fold to three‐fold) of IgM but not IgG titres against echovirus and coxsackievirus. Serological tests for human parvovirus B19, cytomegalovirus, human immunodeficiency virus, and hepatitis C virus were negative. In addition, we ruled out a reactivation of Epstein–Barr virus, or active infections with human herpes virus 6 and adenovirus by quantitative PCR of blood. Finally, biomarkers of autoimmune disorders including RF, p‐ANCA, c‐ANCA, anti‐dsDNA antibody, anti‐PR3 antibody, and anti‐MPO antibody were normal.
Because of the ambiguous serological results, we performed EMB of the left ventricle 9 days after the vaccination. We found no cardiomyocyte hypertrophy, no giant cells, and minimal interstitial fibrosis without proliferating myofibroblast. There were also no signs for haemochromatosis or amyloidosis. Instead, the haematoxylin and eosin stains displayed myocardial oedema and profound mononuclear infiltration in the absence of myocardial necrosis. Immunohistochemistry identified substantial numbers of CD68‐positive macrophages (44 cells/mm2) and CD3‐positive T lymphocytes (15 cells/mm2) (Figure 2 ). Importantly, viral genome analysis of two independent myocardial biopsy samples by quantitative PCR was negative for SARS‐CoV‐2, echovirus, and coxsackievirus.
Figure 2.
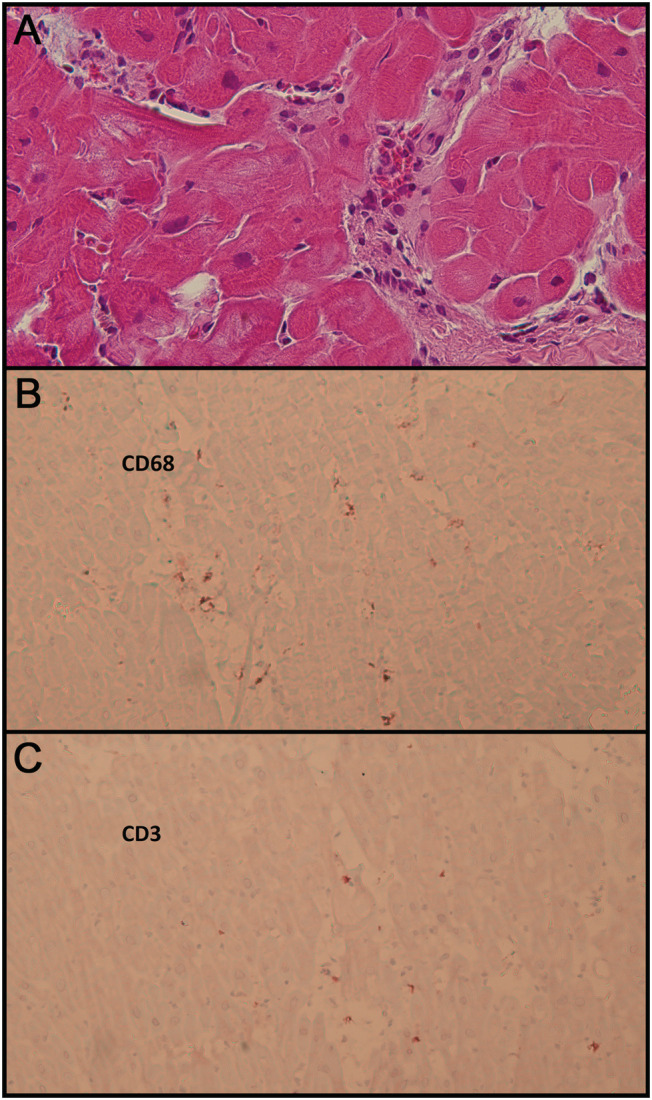
Histology and immunohistology of endomyocardial biopsy. (A) Haematoxylin and eosin stain, primary magnification 40:1. Infiltration of mononuclear inflammatory cells (macrophages and lymphocytes) with mild interstitial oedema. Non‐hypertrophic myofibres with regular nuclei and cytoplasm without degeneration or necrosis. (B) Immunohistochemistry, CD68, anti‐alkaline phosphatase method, primary magnification 20:1. Considerably increased number of macrophages. (C) Immunohistochemistry, CD3, anti‐alkaline phosphatase method, primary magnification 20:1. Moderately increased number of T lymphocytes.
The further clinical course was uneventful. We could discharge the patient soon after having excluded pericardial effusion and mitral valve regurgitation following EMB. Because of the risk of myocarditis and the persistently high antibody titres, we advised the patient against a second vaccine dose. This decision is supported by recent data showing that antibody responses elicited by a single vaccine dose in individuals with prior SARS‐CoV‐2 infection were comparable with those seen after two doses of vaccine in individuals without prior infection. 9
Discussion
We describe a case of acute myocarditis developing within only 12 h after administration of mRNA‐1273. As an advantage to previous reports, 5 , 6 , 7 we were able to confirm and characterize myocarditis by EMB.
Based on the slightly increased IgM titres against echovirus and coxsackievirus, enteroviral infection might be assumed as possible cause of the myocarditis. However, serology has a limited value in the diagnostic of acute enteroviral disease due to cross‐reactivity and poor standardization. 10 Furthermore, whereas enteroviral myocarditis usually starts within 1–4 weeks after a respiratory or gastrointestinal infection and is frequently associated with severe and recurrent symptoms, 10 our patient had been completely asymptomatic for several months. Finally, we ruled out the presence of viral genome in myocardium by EMB. Thus, the role of echovirus and coxsackievirus in this case, if there was any infection, is highly questionable.
Myocarditis has also been suggested as a cardiac complication of COVID‐19 potentially resulting from myocardial viral infection. 11 , 12 , 13 , 14 In most cases, it developed in the setting of fulminant infection with SARS‐CoV‐2 as shown by positive nasopharyngeal swab during pneumonia or acute respiratory distress syndrome. 15 By contrast, our patient repeatedly tested negative for SARS‐CoV‐2, and he had been completely symptom‐free for months until the vaccination. In addition, the fact that PCR analysis of EMB samples was negative for SARS‐CoV‐2 further questions its involvement in the myocardial damage. Thus, the myocarditis presented herein is most likely distinct from SARS‐CoV‐2‐associated myocarditis reported in the literature. 12 , 13 , 15 Because of the detection of IgG against the nucleocapsid of SARS‐CoV‐2, we cannot exclude the theoretical possibility of a delayed onset of myocarditis provoked by a former asymptomatic SARS‐CoV‐2 infection independently of the vaccine. However, because the patient's chest pain occurred within hours after the injection and strictly coincided with other typical signs and symptoms of vaccine reactogenicity, it is reasonable to suggest a causal role for the COVID‐19 vaccine. Whether the previous exposure to SARS‐CoV‐2 antigens in this case may have promoted the development myocarditis during the secondary immune response remains an interesting question.
Because the patient did not show severe cardiac dysfunction, our decision to perform EMB may be controversial. However, the knowledge obtained by EMB was relevant in this case. Considering the patient's former SARS‐CoV‐2 infection, if viral genome had been detected in myocardial tissue, we would have rejected the hypothesis of vaccine adverse effect and there would be no contraindication for the second vaccine dose. In general, the indication for EMB should always be considered with great care because of possible serious complications. Based on the growing evidence of myocarditis associated with COVID‐19 vaccination to date, EMB might be omitted in most cases.
In conclusion, we suggest that myocarditis is a possible side effect of mRNA‐1273. Further research is warranted to elucidate whether this complication is a class effect of mRNA vaccines and to identify populations at higher risk. The knowledge of these issues will not only improve the safety of COVID‐19 vaccines but may also shed light on the pathophysiology of SARS‐CoV‐2‐related myocarditis.
Conflict of interest
None declared.
Nguyen, T. D. , Mall, G. , Westphal, J. G. , Weingärtner, O. , Möbius‐Winkler, S. , and Schulze, P. C. (2021) Acute myocarditis after COVID‐19 vaccination with mRNA‐1273 in a patient with former SARS‐CoV‐2 infection. ESC Heart Failure, 8: 4710–4714. 10.1002/ehf2.13613.
References
- 1. Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID‐19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol 2020; 17: 543–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, Shchendrygina A, Escher F, Vasa‐Nicotera M, Zeiher AM, Vehreschild M, Nagel E. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID‐19). JAMA Cardiol 2020; 5: 1265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ostergaard SD, Schmidt M, Horvath‐Puho E, Thomsen RW, Sorensen HT. Thromboembolism and the Oxford–AstraZeneca COVID‐19 vaccine: side‐effect or coincidence? Lancet 2021; 397: 1441–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Israel examining heart inflammation cases in people who received Pfizer COVID shot. https://www.reuters.com/world/middle‐east/israel‐examining‐heart‐inflammation‐cases‐people‐who‐received‐pfizer‐covid‐shot‐2021‐04‐25/ (13 May 2021; date last accessed).
- 5. Rosner CM, Genovese L, Tehrani BN, Atkins M, Bakhshi H, Chaudhri S, Damluji AA, de Lemos JA, Desai SS, Emaminia A, Flanagan MC, Khera A, Maghsoudi A, Mekonnen G, Muthukumar A, Saeed IM, Sherwood MW, Sinha SS, O'Connor CM, de Filippi CR. Myocarditis temporally associated with COVID‐19 vaccination. Circulation 2021; 144: 502–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Larson KF, Ammirati E, Adler ED, Cooper LT, Hong KN, Saponara G, Couri D, Cereda A, Procopio A, Cavalotti C, Oliva F, Sanna T, Ciconte VA, Onyango G, Holmes DR Jr, Borgeson DD. Myocarditis after BNT162b2 and mRNA‐1273 vaccination. Circulation 2021; 144: 506–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Montgomery J, Ryan M, Engler R, Hoffman D, McClenathan B, Collins L, Loran D, Hrncir D, Herring K, Platzer M, Adams N, Sanou A, Cooper LT Jr. Myocarditis following immunization with mRNA COVID‐19 vaccines in members of the US military. JAMA Cardiol 2021: e212833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meeting highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 7–10 June 2021. https://www.ema.europa.eu/en/news/meeting‐highlights‐pharmacovigilance‐risk‐assessment‐committee‐prac‐7‐10‐june‐2021 (17 July 2021; date last accessed).
- 9. Ebinger JE, Fert‐Bober J, Printsev I, Wu M, Sun N, Prostko JC, Frias EC, Stewart JL, Van Eyk JE, Braun JG, Cheng S, Sobhani K. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS‐CoV‐2. Nat Med 2021; 27: 981–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno‐Blanes J, Felix SB, Fu M, Helio T, Heymans S, Jahns R, Klingel K, Linhart A, Maisch B, McKenna W, Mogensen J, Pinto YM, Ristic A, Schultheiss HP, Seggewiss H, Tavazzi L, Thiene G, Yilmaz A, Charron P, Elliott PM, European Society of Cardiology Working Group on Myocardial and Pericardial Diseases . Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013; 34: 2636–2648 2648a‐2648d. [DOI] [PubMed] [Google Scholar]
- 11. Bearse M, Hung YP, Krauson AJ, Bonanno L, Boyraz B, Harris CK, Helland TL, Hilburn CF, Hutchison B, Jobbagy S, Marshall MS, Shepherd DJ, Villalba JA, Delfino I, Mendez‐Pena J, Chebib I, Newton‐Cheh C, Stone JR. Factors associated with myocardial SARS‐CoV‐2 infection, myocarditis, and cardiac inflammation in patients with COVID‐19. Mod Pathol 2021; 34: 1345–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, Sepe PA, Resasco T, Camporotondo R, Bruno R, Baldanti F, Paolucci S, Pelenghi S, Iotti GA, Mojoli F, Arbustini E. Myocardial localization of coronavirus in COVID‐19 cardiogenic shock. Eur J Heart Fail 2020; 22: 911–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wenzel P, Kopp S, Gobel S, Jansen T, Geyer M, Hahn F, Kreitner KF, Escher F, Schultheiss HP, Munzel T. Evidence of SARS‐CoV‐2 mRNA in endomyocardial biopsies of patients with clinically suspected myocarditis tested negative for COVID‐19 in nasopharyngeal swab. Cardiovasc Res 2020; 116: 1661–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bailey AL, Dmytrenko O, Greenberg L, Bredemeyer AL, Ma P, Liu J, Penna V, Winkler ES, Sviben S, Brooks E, Nair AP, Heck KA, Rali AS, Simpson L, Saririan M, Hobohm D, Stump WT, Fitzpatrick JA, Xie X, Zhang X, Shi PY, Hinson JT, Gi WT, Schmidt C, Leuschner F, Lin CY, Diamond MS, Greenberg MJ, Lavine KJ. SARS‐CoV‐2 infects human engineered heart tissues and models COVID‐19 myocarditis. JACC Basic Transl Sci 2021; 6: 331–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Van Linthout S, Klingel K, Tschope C. SARS‐CoV‐2‐related myocarditis‐like syndromes Shakespeare's question: what's in a name? Eur J Heart Fail 2020; 22: 922–925. [DOI] [PMC free article] [PubMed] [Google Scholar]


