FIGURE 1.
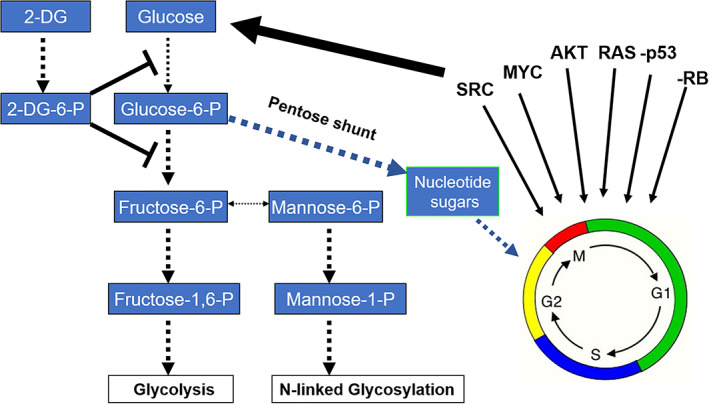
2‐Deoxy‐d‐glucose (2‐DG) exploits cancer‐induced increased glucose uptake and metabolism by blocking glycolysis and interfering with N‐linked glycosylation. The major oncogenes involved in converting a normal cell to a tumor cell have been shown to also upregulate glucose uptake and metabolism. As an analog of glucose, 2‐DG preferentially accumulates in malignant cells and acts to block glycolysis, which supplies the building blocks required for cancer cell replication. Under hypoxia, inhibiting glycolysis leads to cell death, while under normoxia, other energy sources such as fats and amino acids can be used to fuel cells and sustain viability. As an analog of mannose, 2‐DG fraudulently incorporates into the growing oligosaccharide chain required for N‐linked glycosylation leading to unfolded glycoproteins inducing ER stress and activation of PERK (a component of the unfolded protein response [UPR]) which in turn shuts down cap‐dependent translation by phosphorylating EIF2α. We demonstrated that due to 2‐DG's activity as a mannose analog, certain select tumor cell types, even when grown under normoxic conditions, undergo ER stress‐induced UPR‐mediated cell death
