Abstract
Background
Pain has been frequently described as a clinical feature of COVID‐19, and the main pain syndromes that have been associated with the acute phase of this disease so far are headache, myalgia, arthralgia, and neuropathic pain. Understanding the characteristics of pain symptoms is crucial for a better clinical approach.
Methods
Patients who were diagnosed as having COVID‐19 using reverse transcription‐polymerase chain reaction were included in the study. Patients were asked to complete a 51‐item questionnaire via a phone interview, which included questions on demographics, acute COVID‐19 symptoms, the presence of pain symptoms, and their characteristics in the acute phase of COVID‐19.
Results
A total of 222 out of 266 patients with COVID‐19 participated in the study, yielding a response rate of 83.5%. A total of 159 patients reported at least one kind of pain syndrome with a prevalence of 71.6%. Myalgia was reported in 110 (49.6%) patients, headache in 109 (49.1%), neuropathic pain symptoms in 55 (24.8%), and polyarthralgia in 30 (13.5%) patients. A total of 66 patients reported only one type of pain, 46 reported two types, 42 reported three types, and five patients reported all four types of pain. Logistic regression analysis showed that there were significant associations between these pain syndromes and a strong association was found between neuropathic pain and headache.
Conclusion
Pain is a frequently observed symptom of mild‐to‐moderate COVID‐19. There are significant relationships between pain syndromes in COVID‐19, which may be due to a sequence of common etiologic factors.
Significance
This study described the main pain syndromes associated acute phase of mild‐to‐moderate COVID‐19 and its associated features. Headaches and pain of neuropathic characteristics were prevalent in this sample.
1. INTRODUCTION
The number of patients infected by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is increasing globally and has become a global health problem (Director, 2020). The clinical picture of coronavirus disease 2019 (COVID‐19) ranges from common‐cold‐like symptoms to fatal respiratory failure (Guan et al., 2020; Huang et al., 2020). The most common symptoms related to COVID‐19 are fever, fatigue and cough, but available data indicate that pain is also a common symptom (Drożdżal et al., 2020; Murat et al., 2020). The typical pain symptoms associated with COVID‐19 include headache, myalgia, polyarthralgia, and neuropathic pain symptoms (Cipollaro et al.,2020; Drożdżal et al., 2020; Escalera‐Antezana et al., 2020; Mo et al., 2020).
COVID‐19 has been hypothesized to have neuroinvasive potential, with SARS‐CoV‐2 having been found in the cerebrospinal fluid of a few patients (Li, Bai, et al., 2020). SARS‐CoV‐2 binds to angiotensin‐converting enzyme type 2 (ACE‐2) receptors, which are widely expressed in the lungs, heart, and gastrointestinal organs (Hamming et al., 2004). It may also invade the skeletal muscle, vascular smooth muscle, and brain. However, there is still no proof of ACE‐2 receptor expression in the brain, and the SARS‐CoV‐2 neuroinvasive route remains to be elucidated (Ziegler et al., 2020). Moreover, there have been no reports showing the presence of SARS‐CoV‐2 in skeletal muscles or joints. It is therefore still a debate about how the effects of SARS‐CoV‐2 infection on the musculoskeletal system and brain are produced.
Research on pain after COVID‐19 disease is in its infancy. Most of the collected data on pain focused on headache and little is known about overall pain syndromes. A previous study by Soares et al. (2021) reported that patients with COVID‐19 had a higher prevalence of de novo pain (65.2%) and de novo headache (39.1%) when compared with a control group and they found de novo chronic pain in 19.6% of patients with COVID‐19. Another study revealed pain in 69.3% of patients with COVID‐19 with 69.2% of those reporting myalgia/arthralgia, 50.4% headache, 43.6% back pain, and 33.1% reporting low back pain (Murat et al., 2020). Although research has focused on individual pain syndromes, there is still a gap regarding the overall pain symptoms and characteristics, leaving many unresolved questions concerning the characteristics of pain in patients with COVID‐19 (Magdy et al., 2020; Murat et al., 2020; Soares et al., 2021; Uygun et al., 2020). Describing the characteristics of pain syndromes, which may result from different mechanisms, will help to better understand the different mechanisms of pain and will improve the choice of pain medication.
In the current study, we evaluated pain syndromes related to COVID‐19 in the acute phase of COVID‐19, including headache, myalgia, polyarthralgia, and neuropathic pain symptoms, with particular regard to their frequency, severity, and co‐occurrence.
2. METHODS
2.1. Sample selection
This study was conducted in the city of Bursa located in north‐western Turkey. Bursa is the fourth largest city in Turkey with a population of 3,101,833. Some of the asymptomatic and mildly symptomatic patients with COVID‐19 are evaluated in triage tents of some hospitals in the city centre and some infected individuals and their contacts are tested at home visits. Four regional hospitals in the city centre, including our hospital, accept patients with COVID‐19, and patients suspected of having COVID‐19 are evaluated in our emergency outpatient clinic.
Patients who had been admitted to our university hospital emergency department and diagnosed as having COVID‐19 after being analysed using reverse transcription‐polymerase chain reaction (RT‐PCR) with oropharyngeal and nasal swab samples were included in the study. Patient enrolment started in July 2020 and ended in September 2020. Patients were contacted by phone to explain the purpose and design of the study and were asked if they wished to participate. If the first telephone call was unanswered, the patient was phoned for a second time, on a different day and at a different time of day before considering the calls as unsuccessful and no more contact was attempted.
Patients were interviewed about 1.5–3 months after PCR test positivity. Patients with severe disease, cognitively impaired patients, those with a confused state, patients who gave unreliable answers were excluded. Patients aged under 18 years were also excluded from the study. Patients who agreed to participate in the study were interviewed by two experienced neurologists.
2.2. Telephone survey and data collection
The phone survey comprised 51 questions based on four topics, which included demographics, COVID‐19 symptoms, the presence of headache, myalgia, polyarthralgia, neuropathic pain and their characteristics in the acute phase of COVID‐19. Each telephone interview typically lasted between 25 and 40 min. Important characteristics including onset time, duration, and severity of pain were evaluated. Short and clear questions were prepared to sustain the attention of patients during the interviews and to increase the adequacy and responsiveness to the survey.
Initially, the patients were asked about their demographic characteristics and common symptoms of COVID‐19. The patients were questioned on whether they had experienced a headache whilst infected with COVID‐19. If they had, they were questioned about the characteristics of the headache. The previous headaches of patients were classified according to the International Classification of Headache Disorders, 3rd version (ICHD‐3 criteria) (Olesen, 2018).
The patients were then asked whether they experienced new‐onset myalgia, pain with neuropathic characteristics, and polyarthralgia.
New‐onset pain with neuropathic characteristics in the acute phase of COVID‐19 was defined as neuropathic pain symptoms occurring within 30 days of COVID‐19 (Doshi et al., 2021). Tingling, burning, hot, cold or freezing pain, electric shocks or shooting pain evoked by touching and limited to a dermatome or specific neuronal distribution were all accepted as pain with neuropathic characteristics (Krause & Backonja, 2003).
Myalgia was described as pain that was poorly localized or present in a muscle. The patients were asked to state where they felt myalgia most of the time and the location of myalgia was grouped as back pain, extremity pain, and widespread pain. Polyarthralgia was described as pain confined to multiple joints.
The intensity of the myalgia, neuropathic pain symptoms, and polyarthralgia was evaluated using a 10‐point numeric rating scale (1 = ‘no pain at all’ and 10 = ‘the worst pain imaginable’) in response to the question: ‘Think about your previous pain experiences and give a number from 1 to 10 to indicate the intensity of your pain’ (Dalton & McNaull, 1998).
Computed tomography (CT) of the thorax and laboratory tests of the complete blood count, ferritin, creatinine kinase (CK), liver function tests (alanine aminotransferase [ALT], aspartate aminotransferase [AST]) D‐dimer, C‐reactive protein (CRP), lactate dehydrogenase (LDH) and procalcitonin levels were evaluated at admission, and the worst laboratory values during hospitalisation (highest leucocyte, CK, ALT, AST, CRP, LDH, ferritin and procalcitonin and lowest lymphocyte levels) were determined from the patients’ files.
The study protocol was approved by the Local Ethics Committee (Protocol Numbers: 2011‐KAEK‐26/298‐299). The study was performed in accordance with the principles of the Helsinki Declaration.
2.3. Statistical analysis
We included a convenience sample of 220 patients that were tested in our hospital during the main phase of the epidemics. Such sample size allows for a maximum 95% confidence interval of ±6.6% for the proportion of patients with any pain type whatever its prevalence.
Independent sample t‐tests or the Mann–Whitney U test was used to analyse the differences between groups for continuous variables, and two‐sided Fisher's exact, Pearson's chi‐square, and continuity correction tests were used to analyse the differences for categorical variables. Binary logistic regression tests were used with the backward regression variable selection method to explore the differentiating variables between the patient groups, and variables with p < 0.05 in univariate analysis were selected for logistic regression analysis.
The Statistical Package for the Social Sciences Ver. 21.0 software package (IBM SPSS Statistics) was used to calculate statistics. Results were considered to be statistically significant for p‐values ≤0.05.
3. RESULTS
Of the 266 eligible participants, 222 patients were interviewed in the telephone survey, resulting in a response rate of 83.5%. Losses were due to unanswered telephone calls after two attempts on different days and at different times of day, or invalid telephone numbers in 16 (6%) patients, refusal to provide information by telephone in 9 (3.4%) patients, and due to severe disease, confused state or death in 19 (7.1%) patients (Figure 1).
FIGURE 1.
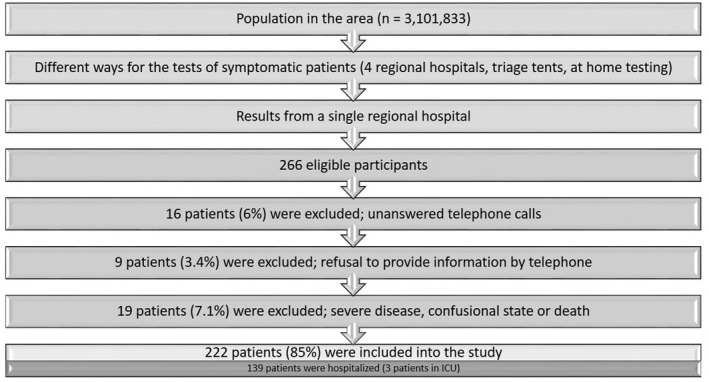
Flowchart of inclusion of the patients with COVID‐19
3.1. The demographics of patients with COVID‐19
The study group comprised 106 (47.7%) females and 116 (52.3%) males. The mean age of the patients was 41.8 ± 14.1 (range, 18–87) years (Table 1). Among the 222 patients, 139 (62.5%) were hospitalized, with three patients having been placed in the intensive care unit (ICU).
TABLE 1.
Demographic characteristics of patients with SARS‐CoV‐2 infection
| All patients (222 patients) | |
|---|---|
| Age (years) (minimum‐maximum) | 41.8 ± 14.1 (18–87) |
| Sex | |
| Female | 106 (47.7%) |
| Current smoker | 36 (16.2%) |
| Comorbidities | |
| Any | 72 (32.4%) |
| Hypertension | 20 (9%) |
| Cardiovascular disease | 9 (4.1%) |
| Endocrine disorders | 6 (2.7%) |
| Diabetes | 14 (6.3%) |
| Respiratory disorders | 6 (2.7%) |
| Malignancy | 4 (1.8%) |
| Number of immune compromised patients | 9 (4.1%) |
| Number of hospitalized patients | 139 (62.5%) |
| Number of patients placed in intensive care unit | 3 (1.4%) |
| Number of patients with COVID‐19 pneumonia a | 104 (46.8%) |
A total of 218 patients underwent chest CT.
The mean length of hospital stay was 9.6 ± 6.3 days. The mean duration of respiratory symptoms was 4.4 ± 2.9 days, 37 (16.7%) patients needed oxygen supplementation, and 177 (79.7%) patients were treated with hydroxychloroquine. Only three (1.4%) patients were admitted to the ICU, one of whom required mechanical ventilation and needed sedation (Table 1).
The number of patients with at least one comorbid disease, such as hypertension, diabetes, cardiovascular or pulmonary disorders, was 72 (32.4%). The number of active smokers was 36 (16.2%) and the number of immune‐compromised patients was nine (4.1%). A total of 218 (98.2%) patients underwent chest CT after admission and a total of 104 (46.8%) patients had COVID‐19 pneumonia.
3.2. Pain in patients with COVID‐19
A total of 159 patients reported at least one kind of pain syndrome with a prevalence of 71.6%. Myalgia was reported in 110 (49.6%) patients, headache in 109 (49.1%), neuropathic pain symptoms in 55 (24.8%), and polyarthralgia in 30 (13.5%) patients (Figure 2). A total of 66 (41.5%) patients reported one type of pain, 46 (28.9%) reported two types, 42 (26.4%) reported three types, and 5 (3.2%) patients reported four types of pain (Figure 2).
FIGURE 2.
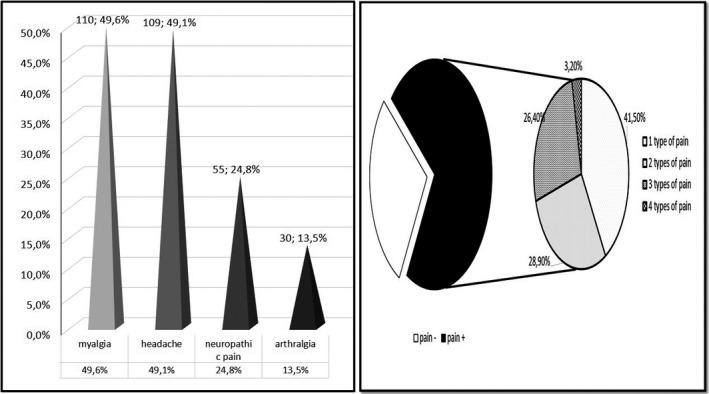
Distribution of pain syndromes in patients with COVID‐19
Analgesic medications were used by 71 (32.0%) participants with COVID‐19, only simple analgesics were used by 55 (77.5%) participants, only non‐steroidal anti‐inflammatory drugs by six (8.4%) patients, and combined analgesics by 10 (14.1%) patients. Three (4.2%) patients required opioids additionally.
3.3. Myalgia and polyarthralgia
Myalgia was detected in 110 (49.6%) patients and polyarthralgia in 30 (13.5%). The mean onset time of myalgia was 2.1 ± 2.2 days, starting on the first day of clinical symptoms in more than half of the patients. The mean duration of myalgia was 11.3 ± 8.4 days and the mean intensity was 7.2 ± 2.0. (Figure 3). The most common pain site was the back in 46 patients (41.8%), followed by widespread pain in 40 (36.4%) patients, and extremity pain in 24 (21.8%) patients.
FIGURE 3.
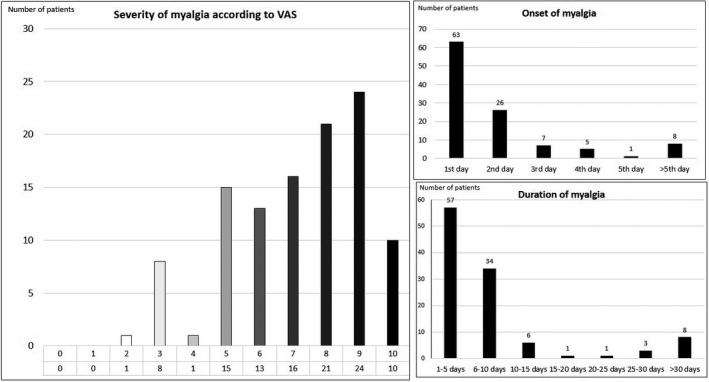
Characteristics of myalgia in patients with COVID‐19
The mean intensity of polyarthralgia was 5.9 ± 1.8.
A comparison of the patients with myalgia is shown in Table 2. Among the investigated parameters, patients with myalgia tended to be older, female, have higher frequencies of fever, sore throat, anosmia, headache, polyarthralgia, and higher levels of procalcitonin at admission, and worse lymphocyte and ferritin levels than patients without myalgia. The binary logistic regression model revealed that the presence of myalgia could be predicted by the following clinical variables: female sex, fever, sore throat, headache, and polyarthralgia (Table 3).
TABLE 2.
Comparison of patients in relation with myalgia and headache
| Myalgia (+) (110) | Myalgia (−) (112) | p (myalgia±) | Headache (+) (109) | Headache (−) (113) | p′ (headache±) | |
|---|---|---|---|---|---|---|
| Age (years) (median [Q3‐Q1]) | 38 (47–30) | 44 (53–30) | 0.04 | 39 (30–49) | 41 (30–52) | NS |
| Female sex | 64 (58.2%) | 42 (37.5%) | 0.02 | 64(58.7%) | 42(37.1%) | 0.01 |
| Hospitalisation | 64 (58.2%) | 75 (67%) | NS | 65 (56.6%) | 74(65.5%) | NS |
| Hospitalisation in ICU | 2 (1.9%) | 1 (0.9%) | NS | 1 (0.9%) | 2(1.8%) | NS |
| Hospitalisation period (days) (median [Q3–Q1]) | 7(12–5) | 6(10–5) | NS | 8(12–6) | 6(10–5) | 0.01 |
| Asthenia | 37 (33.6%) | 25 (22.3%) | NS | 39 (35.8%) | 23 (20.4%) | 0.01 |
| Fever | 63 (57.3%) | 43(38.4%) | 0.05 | 62 (56.9%) | 44 (38.9%) | 0.07 |
| Cough | 63(57.3%) | 51(45.5%) | NS | 67 (61.5%) | 47 (41.6%) | 0.03 |
| Sore throat | 49(44.5%) | 32(28.6%) | 0.01 | 48 (44%) | 33 (29%) | 0.02 |
| Anosmia | 65(59.1%) | 43(38.4%) | 0.02 | 59 (54.1%) | 49 (43.4%) | 0.01 |
| Headache | 69(62.7%) | 40(35.7%) | <0.001 | – | – | – |
| Myalgia | – | – | – | 69 (63.3%) | 41 (36.3%) | <0.001 |
| Arthralgia | 26(23.6%) | 4(3.6%) | <0.001 | 21 (19.3%) | 9 (8%) | 0.02 |
| Neuropathic pain | 35(31.8%) | 20(17.9%) | 0.012 | 43 (39.4%) | 12 (10.6%) | <0.001 |
| Comorbid disease | 31(28.2%) | 41(36.6%) | NS | 37 (33.9%) | 35 (31%) | NS |
| Smoking | 17(15.7%) | 19(17.4%) | NS | 14 (12.8%) | 22 (20.4%) | NS |
| Immuncompromised states | 4(3.8%) | 5(4.5%) | NS | 4 (3.7%) | 5(4.6%) | NS |
| Pneumonia | 49(45.4%) | 55(50.0%) | NS | 56 (51.9%) | 48 (43.6%) | NS |
| Laboratory findings median (Q3–Q1) | ||||||
| Leukocyte value at admission(K/µl) | 6190.0 (8002.5–4817.0) | 6855.0 (8530.0–5390.0) | NS | 6200.0 (8470.0–4990.0) | 6890.0 (8375.0–4920.0) | NS |
| Worst leukocyte value(K/µl) | 7205.0 (9262.5–5705.0) | 7630.0 (9090.0–6227.5) | NS | 7380.0 (9120.0–5830.0) | 7480.0 (9230.0–6027.0) | NS |
| Lymphocyte value at admission(K/µl) | 1746.5 (2307.3–1085.5) | 1864.0 (2605.5–1219.8) | NS | 1723.0 (6310.0–1130.0) | 1880.0 (7020.0–100.0) | NS |
| Worst lymphocyte value(K/µl) | 1370.5 (1940.8–950.0) | 1631.0 (2317.0–1086.3) | 0.04 | 1379.0 (2029.0–990.0) | 1606.0 (2295.0–1021.0) | NS |
| Ferritin level (µg/L) | 68.8 (150.2–25.3) | 125.6 (226.0–42.0) | 0.006 | 53.8 (157.8–21.6) | 123.0 (203.5–62.5) | 0.01 |
| Worst ferritin level (µg/L) | 90.0 (192.2–35.0) | 142.0 (296.0–56.1) | NS | 88.0 (233.9–31.5) | 139.6 (256.3–62.5) | 0.02 |
| CRP level at admission (mg/L) | 2.4 (7.1–2.0) | 3.7 (16.8–2) | NS | 2.5 (8.8–2.0) | 3.4 (13.3–2.0) | NS |
| Worst CRP level (mg/L) | 4.2 (18.0–2.0) | 5.1 (37.8–2.0) | NS | 5.2 (26.8–2.0) | 4.2 (20.0–2.0) | NS |
| CK at admission (U/L) | 69.0 (99.0–48.5) | 72.5 (99.3–51.8) | NS | 72.0 (95.5–49.0) | 71.5 (100.3–52.0) | NS |
| Worst CK value (U/L) | 75.0 (116.5–52.0) | 79.5 (112–52.8) | NS | 76.0 (112.0–51.0) | 80.0 (116.0–53.0) | NS |
| D‐dimer (mg/ml) | 0.3 (0.6–0.2) | 0.3 (0.6–0.2) | NS | 0.3 (0.6–0.2) | 0.3 (0.6–0.2) | NS |
| Worst D‐dimer value (mg/ml) | 0.4 (0.7–0.3) | 0.4 (0.3–0.8) | NS | 0.5 (0.8–0.3) | 0.4 (0.7–0.3) | NS |
| Procalcitonin (ng/ml) | 0.1 (0.1–0.0) | 0.1(0.2–0.1) | 0.027 | 0.1 (0.1–0.1) | 0.02 (0.1–0.1) | NS |
| Worst procalcitonin(ng/ml) | 0.1 (0.1–0.0) | 0.1(0.1–0.0) | NS | 0.1 (0.1–0.1) | 0.1(0.1–0.1) | NS |
| LDH value (U/L) | 193.0 (234.0–163.0) | 215.0 (271.5–184.0) | NS | 204.5 (267.5–171.3) | 198.0 (241.0–167.3) | NS |
| Worst LDH value (U/L) | 209.0 (258.0–179.0) | 227.0 (273.0–191.0) | NS | 227.5 (269.5–186.8) | 213.0 (263.8–179.0) | NS |
| AST value (U/L) | 20.0 (26.3–18.0) | 21.0 (26.0–17.0) | NS | 21.0 (27.0–17.0) | 21.0 (26.0–18.0) | NS |
| Worst AST value (U/L) | 24.0 (35.3– 20.0) | 24.0 (37.5–19.0) | NS | 25.0 (37.0–20.0) | 24.0 (36.0–19.0) | NS |
| ALT value (U/L) | 21.0 (29.3–16.0) | 20.0 (30.5–15.0) | NS | 20.0 (29.0–15.0) | 21.0 (33.0–15.3) | NS |
| Worst ALT value (U/L) | 25.0 (46.0–17.8) | 27.0 (51.5–16.5) | NS | 29.0 (46.0–19.0) | 24.5 (52.0–16.3) | NS |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CK, creatinine kinase; CRP, c‐reactive protein; LDH, lactate dehydrogenase; Q1, first quartile; Q3, third quartile. Please note that interquartile range is IQR = Q3‐Q1.
Bold indicates statistical significance.
TABLE 3.
Significant variables of myalgia using backward stepwise logistic regression analysis
| Variable | Wald | OR | 95% CI | p | |
|---|---|---|---|---|---|
| Female gender | 0.691 | 1.995 | 1.031 | 3.859 | 0.040 |
| Fever | 0.751 | 2.119 | 1.101 | 4.077 | 0.025 |
| Sore throat | 0.751 | 2.120 | 1.088 | 4.131 | 0.027 |
| Headache | 0.893 | 2.443 | 1.262 | 4.730 | 0.008 |
| Arthralgia | 1.895 | 6.653 | 2.039 | 21.704 | 0.002 |
Variable(s) entered on step 1: age, female sex, higher frequency of fever, sore throat, anosmia, headache, arthralgia, procalcitonin and ferritin levels at admission and worse lymphocyte levels.
Bold indicates statistical significance.
3.4. Headache
The characteristics of headaches in the patients with COVID‐19 are shown in Table 4. The mean onset time of headache was 1.7 ± 3.0 days and headache started on the first day of symptoms in 68 out of 109 patients (62.4%). Most (83.5%) of the patients had a moderate or severe headache and 58.7% of the patients with headache described it as pulsating.
TABLE 4.
Headache characteristics of patients with COVID‐19
| Headache onset day | |
| 1st day | 68 (62.4%) |
| 2nd day | 15 (13.8%) |
| 3rd day | 8 (7.3%) |
| 4th day | 5 (4.6%) |
| 5th day | 2 (1.8%) |
| >5 days | 11 (10.1%) |
| Headache severity | |
| Mild | 18 (16.5%) |
| Moderate | 64 (58.7%) |
| Severe | 27 (24.8%) |
| Headache characteristics | |
| Pulsating | 64 (58.7%) |
| Pressing | 31 (28.4%) |
| Fiery | 8 (7.3%) |
| Stabbing | 9 (8.3%) |
| Other | 14 (12.8%) |
| Localisation | |
| Only unilateral | 9 (8.3%) |
| Only bilateral | 97 (88.9%) |
| Bilateral predominant one side | 3 (2.8%) |
| Accompanying symptoms | |
| Nausea/Vomitting | 39 (35.8%) |
| Photophobia | 24 (22%) |
| Phonophobia | 29 (26.6%) |
| Osmophobia | 3 (2.8%) |
| Anosmia | 5 (4.5%) |
| Aggravation with movement | 38 (34.9%) |
Eighty‐one patients reported experiencing headaches before the infection and 69 (85.2%) stated that their headache whilst infected was different from previous ones. Forty‐seven patients reported having a higher headache frequency with COVID‐19 than without and 30 of the subjects with headache described it as more severe than before being infected with COVID‐19. Twenty‐eight (25.7%) patients reported that infection resulted in new‐onset headache (Figure 4).
FIGURE 4.
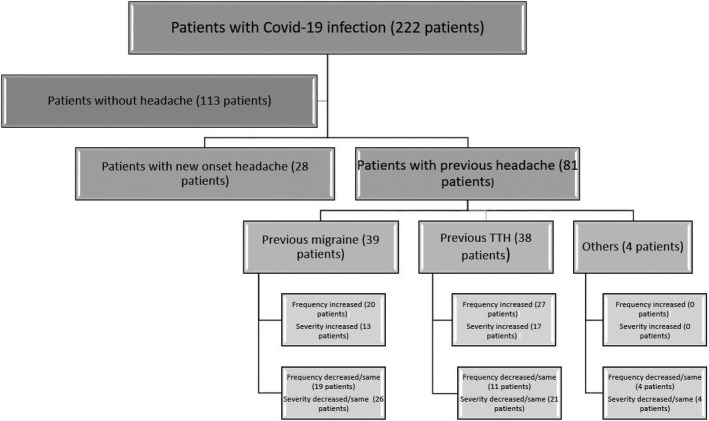
Distribution of headache in patients with COVID‐19
A comparison of the patients with and without headache is shown in Table 2. The following clinical variables: female sex, myalgia and neuropathic pain symptoms were found to be significant risk factors for the presence of headache in the logistic regression model (Table 5).
TABLE 5.
Significant variables of headache using backwards stepwise logistic regression analysis
| Wald | OR | 95% CI | p | ||
|---|---|---|---|---|---|
| Female | 3.893 | 1.934 | 1.004 | 3.723 | 0.048 |
| Cough | 3.187 | 1.811 | 0.944 | 3.476 | 0.074 |
| Myalgia | 6.112 | 2.339 | 1.193 | 4.587 | 0.013 |
| Arthralgia | 2.922 | 2.571 | 0.871 | 7.591 | 0.087 |
| Neuropathic pain | 15.721 | 5.427 | 2.352 | 12.522 | <0.001 |
Variable(s) entered on step 1: female sex, higher frequencies of asthenia, fever, cough, sore throat, anosmia, myalgia and arthralgia, neuropathic pain, ferritin levels at admission and worst ferritin level.
Bold indicates statistical significance.
3.5. Characteristics of neuropathic pain symptoms
Neuropathic pain symptoms were detected in 55 (24.8%) patients infected with COVID‐19. The mean intensity of neuropathic pain was 5.8 with a standard deviation of 2.4.
Numbness was the most commonly reported neuropathic pain symptom followed by burning pain and tingling pain (Figure S1). The most frequent locations were the extremities in 47 (85.5%) patients, followed by back pain in five (9.1%) and neck pain in three (5.5%) patients.
Patients with pain with neuropathic pain characteristics tended to be female, have higher frequencies of asthenia, cough, sore throat, anosmia, headache and myalgia, and have higher ferritin levels at admission than patients without neuropathic pain. Binary logistic regression analysis demonstrated that having a sore throat and headache had a statistically significant effect on the presence of neuropathic pain symptoms (Tables S1 and S2). The most significant comorbid condition was headache.
4. DISCUSSION
In the present study, we investigated pain syndromes in patients with acute COVID‐19 and found that 71.6% of patients had at least one type of pain. Among the investigated pain conditions, the most common was myalgia (49.6%), followed by headache (49.1%), neuropathic pain symptoms (24.8%), and polyarthralgia (13.5%). Our study may support that there are significant associations between these pain syndromes, which could be due to a sequence of common etiologic pathways. Additionally, apart from the infection itself, the isolation of patients can cause stress and anxiety, which may lead to a worsening of painful symptoms.
The underlying pathophysiology of acute pain in patients with COVID‐19 has not been elucidated. Prolonged immobilisation, sedation, loss of cardiopulmonary performance, risks of procedural pain, and use of medications including corticosteroids and neuromuscular blocking agents in ICUs, and prolonged mechanical ventilation may indirectly cause pain in the acute phase of COVID‐19 (Kemp et al., 2020; Moisset et al., 2021). However, we found no link between pain syndromes and hospitalisation in our sample. Only three patients were admitted to the ICU and only one required mechanical ventilation and this patient reported three types of pain including headache, myalgia, and neuropathic pain symptoms. It is difficult to comment on severe cases of COVID‐19 in our sample because our population mostly included mild‐moderate cases of COVID‐19. Psychological factors such as social isolation, and posttraumatic stress disorder (PTSD) or neurologic complications of the infection may also be potential risk factors for the development of pain. Apart from these indirect effects, a consequence of a direct viral invasion may also be accounted for pain in SARS‐CoV2 infection (Disser et al., 2020). The SARS‐CoV‐2 virus can spread through vessels and cause infections in tissues containing ACE‐2. The binding of SARS‐CoV‐2 to the ACE‐2 receptor in skeletal muscle, vascular smooth muscle, and brain, which leads to a decrease in ACE‐2, accounted for ACE‐2 mediated neurotoxicity, neuroinflammation, and neurodegeneration (Baptista et al., 2020; Hoffmann et al., 2020; Kucuk et al., 2020; Pennisi et al., 2020; Su et al., 2020, Weng et al., 2020; Zhang et al., 2020). According to Garvin et al. (2020), SARS‐CoV‐2 reduces the levels of ACE in lung cells whilst increasing the levels of ACE‐2 and this may lead to ‘bradykinin storm,’ which could induce pain in COVID‐19. Also, SARS‐CoV‐2 infection can potentially lead to neuromuscular symptoms through a molecular mimicry mechanism (cross‐reacting epitope between the virus and the host) (Shah et al., 2020).
4.1. Myalgia and polyarthralgia
Our study indicated that the most frequent pain symptom in patients with COVID‐19 was myalgia. Previous studies also found myalgia to be one of the most common symptoms in up to 50% of patients (Kucuk et al., 2020; Li, Huang, et al., 2020; Murat et al., 2020). However, the frequency of myalgia and arthralgia varies widely between studies, and the severity and duration of myalgia have not yet been investigated in detail. Other symptoms associated have also not been addressed. We found moderate or severe pain with a mean duration of 11 days and a mean onset time of 2 days to be important characteristics of myalgia in patients with COVID‐19. The predictive factors for myalgia were female sex, fever, sore throat, headache, and arthralgia. Our study also showed that myalgia was not associated with pneumonia, hospitalisation, infection parameters or muscle enzymes. Both our findings and previous reports suggest that myalgia may not be a predictor of contracting a severe case of COVID‐19 (Lippi et al., 2020). Muscle pain was not associated with high levels of CK and LDH, supporting the notion that this symptom is not directly accounted for by muscle injury.
Arthralgia is a common pain syndrome in viral infections (Widyadharma et al., 2020). Joint pain accompanying myalgia has been frequently reported by patients with COVID‐19, but only rarely has arthralgia been reported at admission (Joob & Wiwanitkit, 2020; Vacchiano et al., 2020). In line with these findings, our study showed that 13.5% of patients with COVID‐19 had arthralgia and that it was more frequently reported by patients with myalgia.
4.2. Headache
Headache was the second most frequently detected pain symptom in our study (49.1%). Headaches in patients with COVID‐19 were primarily reported to be bilateral, of moderate intensity, and pulsating. The mean onset time of headache was 1.7 days with a mean duration of 2–4 days. Many other studies have also found headache to be a frequent symptom of COVID‐19, with a prevalence ranging from 6%–78% (García‐Azorín et al., 2020; Mao et al., 2020; Membrilla et al., 2020; Poncet‐Megemont et al., 2020; Uygun et al., 2020; Wu et al., 2020). Similar to our study, Poncet‐Megemont et al. (2020) showed that 59% of 139 consecutive patients had a new‐onset headache during the acute phase of COVID‐19.
The majority of the patients who had previously had headaches reported a change in the characteristics and an increase in the frequency of headaches whilst infected with COVID‐19. On the other hand, the severity of headaches was reported to be the same or even less by over half of the patients in our study population. The apparent discrepancy between the severity and frequency of headaches in patients with COVID‐19 may be due to individual differences in perception of pain.
According to our results, patients with COVID‐19 with headache often have other types of pain syndromes, including myalgia, arthralgia, and neuropathic pain symptoms. The pathophysiologic mechanisms causing the strong relationship between these pain syndromes in patients with COVID‐19 is not clear but it is presumably associated with generalized inflammation and cytokine response, which results in nociceptive activation and central sensitisation or indirect effects of COVID‐19 such as social isolation, stress, anxiety, and PTSD, as mentioned (Hoffmann et al., 2020; Pennisi et al., 2020; Zhang et al., 2020).
Our findings indicate that pneumonia, hospitalisation, and laboratory findings are not associated with the presence of headache in patients with COVID‐19.
4.3. Neuropathic pain symptoms
Neuropathic pain symptoms were detected in nearly 25% of patients infected with SARS‐CoV‐2 in our study group. This was higher than that found in a previous observational study, which stated that only 2.3% of hospitalized patients with COVID‐19 experienced neuropathic pain (Mao et al., 2020). This disparity may be a result of the adverse effects of medications such as hydroxychloroquine, age, comorbidities, or the immobility of the patients.
Numbness was the most commonly reported neuropathic pain symptom followed by burning pain and squeezing pain in patients with COVID‐19 in our study group. Describing the characteristics of neuropathic pain in patients with COVID‐19 can help to understand the different mechanisms of pain. Moreover, understanding the characteristics of neuropathic pain, which may result from different mechanisms, will improve the choice of pain medication (von Hehn et al., 2012).
4.4. Sex differences in pain
Several studies have revealed that pain is generally more frequent in females (Abraham et al., 2018; Mogil, 2012)—a result that our study also supports. Possible explanations for this disparity between the sexes range from genetic and hormonal differences to psychosocial factors (Abraham et al., 2018; Mogil, 2012). The immune system also plays a major role in the development of pain. With infection, the activation of immune cells triggers an inflammatory reaction, which sensitises nerve fibres and results in pain. It is thought that due to having more oestrogen, the excitability of sensory nerves is increased in females (Berkley et al., 2006).
4.5. Limitations and strengths of the study
This study has some limitations, the major one being that it is a study in which the survey was performed by self‐report, meaning that the patients’ responses may have been affected by recall bias. Secondly, pain is a multifactorial and complex experience; surveys may not reflect the full impact of pain on patients adequately. Except for headache, only new‐onset pain syndromes reported by the patients were evaluated in our study and it is a limitation that patients with previous chronic pain syndromes were not excluded. The report from patients with chronic pain who already had neuropathic pain and/or arthralgia may lead to bias in the findings related to these pain syndromes; previous pain conditions may significantly influence the risk of development of new‐onset pain during COVID‐19. Comorbid conditions such as depression and cardiovascular disease were not evaluated in this study.
Another limitation of this study is that asymptomatic patients and patients with mild symptoms who are were tested in tents of hospitals in the city centre and home visits and severe patients did not participate in the study, which limits the generalizability of the results to other settings. We did not focus on other types of pain related to COVID‐19 such as sore throat, chest and abdominal pain, and procedural pain induced by nasopharyngeal swab (Moisset et al., 2021; Weng et al., 2021). Also, pain onset time for headache had no missing data, but there were missing data for pain onset time for other pain conditions and the chronologic data.
A further limitation of this survey is that the assessment of neuropathic pain symptoms of participants was conducted by using a telephone survey, which can be biased in numerous respects. Neuropathic pain symptoms were evaluated using a neuropathic pain questionnaire including 12 questions regarding pain and does not include neurologic examination and visual contents, which makes it suitable for telephone interviews (Krause & Backonja, 2003). One other limitation of our study is that it does not point to a particular type of neuropathy as a cause of neuropathic pain (e.g. polyneuropathy, radiculopathy, and myelopathy). Additionally, we did not evaluate the presence of allodynia and objective signs of arthritis.
One other major limitation of our study is that we did not include a control group, which limits conclusions about how the pain phenomenon and its mechanism differs from other infections of similar severity. A recent study by Soares et al. compared patients discharged from hospital after SARS‐CoV‐2 infections with patients hospitalized at the same time and found that patients with COVID‐19 had a higher prevalence of de novo pain when compared with the control group, which is an original finding (65.2% and 11.0%, respectively). However, the authors did not characterize the clinical features and predictors of pain in the study (Soares et al., 2021).
Our study also has strengths, namely, that all patients were surveyed by experienced neurologists and all neurologic pain syndromes were evaluated using a detailed questionnaire, which evaluated the frequency, severity, and types of pain, meticulously. Moreover, the laboratory findings and imaging results were evaluated from the patients’ files in detail.
5. CONCLUSION
Pain is a frequently observed symptom of mild‐to‐moderate COVID‐19 and pain syndromes including myalgia, polyarthralgia, headache and neuropathic pain symptoms are strongly interlinked. The reason for this may be a sequence of common etiologic factors. Our understanding of these pathways will improve as more is discovered about the pathophysiology of COVID‐19, and prospective studies are needed to confirm the associations between pain syndromes in COVID‐19.
CONFLICT OF INTEREST
None of the authors has any conflict of interest. This work, either in part or full, is not presented previously.
AUTHOR CONTRIBUTIONS
EOA, GG, EK, NK extracted and synthesized the data. All authors were involved in interpreting the data. EOA and NK drafted the manuscript. All authors revised it critically for important intellectual content and approved the final version of the manuscript for submission.
Supporting information
Fig S1
Table S1‐S2
Supplementary Material
1.
Pandemic Study Team
Halis Akalın, MD; Mustafa Hacı Mustafaoglu, MD; Erol Armagan, MD; Cagri Hunutlu, MD; Aynur Urhan, MD; Esra Kazak, MD; Yasemin Heper, MD; Mehmet Karadag, MD; Funda Coskun, MD; Esra Uzaslan, MD; Dane Ediger, MD; Asli Dilek Gorektasli, MD; Ezgi Demirdogen, MD; Nilufer Aylin Acet‐Ozturk, MD; Fahir Ozkalemkas, MD; Solmaz Celebi, MD; Gurkan Uncu, MD; Alpaslan Turkkan, MD; Alis Ozcakir, MD; Levent Ozdemir, MD; Cuneyt Ozakın, MD; Nermin Kelebek, MD; Fatma Duzgun, MSc; Nevin Bor, MSc; Sevginar Sakarya, MSc; Fahri Durmaz, MSc; Mufit Parlak, MD; Mustafa Gullulu, MD; Suna Goren, MD; Ali Aydinlar, MD; Kemal Durak, MD; Remzi İscimen, MD; Bedrettin Akova, MD; Saduman Balaban Adim, MD; Suheda Ozcakir, MD; Ayse Melda Payaslioglu, MD; Haluk Barbaros Oral, MD; Ekrem Kaya, MD; Irfan Kiristioglu, MD; Ridvan Ali, MD.
Uludag School of Medicine, Uludag University, Bursa, Turkey.
Oguz‐Akarsu, E. , Gullu, G. , Kilic, E. , Dinç, Y. , Ursavas, A. , Yilmaz, E. , Zarifoglu, M. , & Karli, N. ; Pandemic Study Team (2022). Insight into pain syndromes in acute phase of mild‐to‐moderate COVID‐19: Frequency, clinical characteristics, and associated factors. European Journal of Pain, 26, 492–504. 10.1002/ejp.1876
This accompanies the following article: Kubota, GT & Moisset, X. What lies behind and beyond acute COVID‐19 pain?. Eur J Pain. 2022; 26: 282–283. https://doi.org/10.1002/ejp.1889
Contributor Information
Emel Oguz‐Akarsu, Email: emeloguz@yahoo.com.
Pandemic Study Team:
Halis Akalın, Mustafa Hacı Mustafaoglu, Erol Armagan, Cagri Hunutlu, Aynur Urhan, Esra Kazak, Yasemin Heper, Mehmet Karadag, Funda Coskun, Esra Uzaslan, Dane Ediger, Asli Dilek Gorektasli, Ezgi Demirdogen, Nilufer Aylin Acet‐Ozturk, Fahir Ozkalemkas, Solmaz Celebi, Gurkan Uncu, Alpaslan Turkkan, Alis Ozcakir, Levent Ozdemir, Cuneyt Ozakın, Nermin Kelebek, Fatma Duzgun, Nevin Bor, Sevginar Sakarya, Fahri Durmaz, Mufit Parlak, Mustafa Gullulu, Suna Goren, Ali Aydinlar, Kemal Durak, Remzi İscimen, Bedrettin Akova, Saduman Balaban Adim, Suheda Ozcakir, Ayse Melda Payaslioglu, Haluk Barbaros Oral, Ekrem Kaya, Irfan Kiristioglu, and Ridvan Ali
REFERENCES
- Abraham, A. , Barnett, C. , Katzberg, H. D. , Lovblom, L. E. , Perkins, B. A. , & Bril, V. (2018). Sex differences in neuropathic pain intensity in diabetes. Journal of the Neurological Sciences, 388, 103–106. 10.1016/j.jns.2018.03.008 [DOI] [PubMed] [Google Scholar]
- Baptista, A. F. , Baltar, A. , Okano, A. H. , Moreira, A. , Campos, A. C. P. , Fernandes, A. M. , Brunoni, A. R. , Badran, B. W. , Tanaka, C. , de Andrade, D. C. , da Silva Machado, D. G. , Morya, E. , Trujillo, E. , Swami, J. K. , Camprodon, J. A. , Monte‐Silva, K. , Sá, K. N. , Nunes, I. , Goulardins, J. B. , … Zana, Y. (2020). Applications of non‐invasive neuromodulation for the management of disorders related to COVID‐19. Frontiers in Neurology, 11, 1248. 10.3389/fneur.2020.573718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkley, K. J. , Zalcman, S. S. , & Simon, V. R. (2006). Sex and gender differences in pain and inflammation: A rapidly maturing field. American Journal of Physiology‐Regulatory, Integrative and Comparative Physiology, 291(2), R241–R244. 10.1152/ajpregu.00287.2006 [DOI] [PubMed] [Google Scholar]
- Cipollaro, L. , Giordano, L. , Padulo, J. , Oliva, F. , & Maffulli, N. (2020). Musculoskeletal symptoms in SARS‐CoV‐2 (COVID‐19) patients. Journal of Orthopaedic Surgery and Research, 15(1), 178. 10.1186/s13018-020-01702-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton, J. A. , & McNaull, F. (1998). A call for standardizing the clinical rating of pain intensity using a 0 to 10 rating scale. Cancer Nursing, 21(1), 46–49. 10.1097/00002820-199802000-00006 [DOI] [PubMed] [Google Scholar]
- Director, W. (2020). General's opening remarks at the media briefing on COVID‐19‐11 March 2020. World Health Organization. [Google Scholar]
- Disser, N. P. , De Micheli, A. J. , Schonk, M. M. , Konnaris, M. A. , Piacentini, A. N. , Edon, D. L. , Toresdahl, B. G. , Rodeo, S. A. , Casey, E. K. , & Mendias, C. L. (2020). Musculoskeletal consequences of COVID‐19. Journal of Bone and Joint Surgery, 102, 1197–1204. 10.2106/JBJS.20.00847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doshi, T. L. , Dworkin, R. H. , Polomano, R. C. , Carr, D. B. , Edwards, R. R. , Finnerup, N. B. , Freeman, R. L. , Paice, J. A. , Weisman, S. J. , & Raja, S. N. (2021). AAAPT diagnostic criteria for acute neuropathic pain. Pain Medicine, 22(3), 616–636. 10.1093/pm/pnaa407 [DOI] [PubMed] [Google Scholar]
- Drożdżal, S. , Rosik, J. , Lechowicz, K. , Machaj, F. , Szostak, B. , Majewski, P. , Rotter, I. , & Kotfis, K. (2020). COVID‐19: Pain management in patients with SARS‐CoV‐2 infection‐molecular mechanisms, challenges, and perspectives. Brain Sciences, 10(7), 465. 10.3390/brainsci10070465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalera‐Antezana, J. P. , Lizon‐Ferrufino, N. F. , Maldonado‐Alanoca, A. , Alarcón‐De‐la‐Vega, G. , Alvarado‐Arnez, L. E. , Balderrama‐Saavedra, M. A. , Bonilla‐Aldana, D. K. , Rodríguez‐Morales, A. J. , & LANCOVID . (2020). Clinical features of the first cases and a cluster of Coronavirus Disease 2019 (COVID‐19) in Bolivia imported from Italy and Spain. Travel Medicine and Infectious Disease, 35, 101653, 10.1016/j.tmaid.2020.101653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García‐Azorín, D. , Trigo, J. , Talavera, B. , Martínez‐Pías, E. , Sierra, Á. , Porta‐Etessam, J. , Arenillas, J. F. , & Guerrero, Á. L. (2020). Frequency and type of red flags in patients with Covid‐19 and headache: A series of 104 hospitalized patients. Headache, 60(8), 1664–1672. 10.1111/head.13927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvin, M. R. , Alvarez, C. , Miller, J. I. , Prates, E. T. , Walker, A. M. , Amos, B. K. , Mast, A. E. , Justice, A. , Aronow, B. , & Jacobson, D. (2020). A mechanistic model and therapeutic interventions for COVID‐19 involving a RAS‐mediated bradykinin storm. eLife, 9, e59177. 10.7554/eLife.59177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, W. J. , Ni, Z. Y. , Hu, Y. , Liang, W. H. , Ou, C. Q. , He, J. X. , Liu, L. , Shan, H. , Lei, C. L. , Hui, D. , Du, B. , Li, L. J. , Zeng, G. , Yuen, K. Y. , Chen, R. C. , Tang, C. L. , Wang, T. , Chen, P. Y. , Xiang, J. , … China Medical Treatment Expert Group for Covid‐19 . (2020). Clinical characteristics of coronavirus disease 2019 in China. The New England Journal of Medicine, 382(18), 1708–1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming, I. , Timens, W. , Bulthuis, M. L. , Lely, A. T. , Navis, G. , & van Goor, H. (2004). Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. The Journal of Pathology, 203(2), 631–637. 10.1002/path.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, M. , Kleine‐Weber, H. , Schroeder, S. , Krüger, N. , Herrler, T. , Erichsen, S. , Schiergens, T. S. , Herrler, G. , Wu, N. H. , Nitsche, A. , Müller, M. A. , Drosten, C. , & Pöhlmann, S. (2020). SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell, 181(2), 271–280.e8. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, C. , Wang, Y. , Li, X. , Ren, L. , Zhao, J. , Hu, Y. I. , Zhang, L. I. , Fan, G. , Xu, J. , Gu, X. , Cheng, Z. , Yu, T. , Xia, J. , Wei, Y. , Wu, W. , Xie, X. , Yin, W. , Li, H. , Liu, M. , … Cao, B. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England), 395(10223), 497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joob, B. , & Wiwanitkit, V. (2020). Arthralgia as an initial presentation of COVID‐19: Observation. Rheumatology International, 40(5), 823. 10.1007/s00296-020-04561-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp, H. I. , Corner, E. , & Colvin, L. A. (2020). Chronic pain after COVID‐19: Implications for rehabilitation. British Journal of Anaesthesia, 125(4), 436–440. 10.1016/j.bja.2020.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause, S. J. , & Backonja, M. M. (2003). Development of a neuropathic pain questionnaire. The Clinical Journal of Pain, 19(5), 306–314. 10.1097/00002508-200309000-00004 [DOI] [PubMed] [Google Scholar]
- Kucuk, A. , Cumhur Cure, M. , & Cure, E. (2020). Can COVID‐19 cause myalgia with a completely different mechanism? A hypothesis. Clinical Rheumatology, 39(7), 2103–2104. 10.1007/s10067-020-05178-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L. Q. , Huang, T. , Wang, Y. Q. , Wang, Z. P. , Liang, Y. , Huang, T. B. , Zhang, H. Y. , Sun, W. , & Wang, Y. (2020). COVID‐19 patients’ clinical characteristics, discharge rate, and fatality rate of meta‐analysis. Journal of Medical Virology, 92(6), 577–583. 10.1002/jmv.25757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. C. , Bai, W. Z. , & Hashikawa, T. (2020). The neuroinvasive potential of SARS‐CoV2 may play a role in the respiratory failure of COVID‐19 patients. Journal of Medical Virology, 92(6), 552–555. 10.1002/jmv.25728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi, G. , Wong, J. , & Henry, B. M. (2020). Myalgia may not be associated with severity of coronavirus disease 2019 (COVID‐19). World Journal of Emergency Medicine, 11(3), 193–194. 10.5847/wjem.j.1920-8642.2020.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magdy, R. , Hussein, M. , Ragaie, C. , Abdel‐Hamid, H. M. , Khallaf, A. , Rizk, H. I. , & Dahshan, A. (2020). Characteristics of headache attributed to COVID‐19 infection and predictors of its frequency and intensity: A cross sectional study. Cephalalgia, 40(13), 1422–1431. 10.1177/0333102420965140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, L. , Jin, H. , Wang, M. , Hu, Y. , Chen, S. , He, Q. , Chang, J. , Hong, C. , Zhou, Y. , Wang, D. , Miao, X. , Li, Y. , & Hu, B. (2020). Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurology, 77(6), 683–690. 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Membrilla, J. A. , de Lorenzo, Í. , Sastre, M. , & Díaz de Terán, J. (2020). Headache as a cardinal symptom of coronavirus disease 2019: A cross‐sectional study. Headache, 60(10), 2176–2191. 10.1111/head.13967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo, P. , Xing, Y. , Xiao, Y. , Deng, L. , Zhao, Q. , Wang, H. , Xiong, Y. , Cheng, Z. , Gao, S. , Liang, K. , Luo, M. , Chen, T. , Song, S. , Ma, Z. , Chen, X. , Zheng, R. , Cao, Q. , Wang, F. , & Zhang, Y. (2020). Clinical characteristics of refractory COVID‐19 pneumonia in Wuhan, China. Clinical Infectious Diseases, ciaa270. Advance online publication. 10.1093/cid/ciaa270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil, J. S. (2012). Sex differences in pain and pain inhibition: Multiple explanations of a controversial phenomenon. Nature Reviews Neuroscience, 13(12), 859–866. 10.1038/nrn3360 [DOI] [PubMed] [Google Scholar]
- Moisset, X. , Gautier, N. , Godet, T. , Parabère, S. , Pereira, B. , Meunier, E. , Gerbaud, L. , Lesens, O. , Henquell, C. , Beytout, J. , & Clavelou, P. (2021). Nasopharyngeal swab‐induced pain for SARS‐CoV‐2 screening: A randomised controlled trial of conventional and self‐swabbing. European Journal of Pain, 25(4), 924–929. 10.1002/ejp.1722 [DOI] [PubMed] [Google Scholar]
- Murat, S. , Dogruoz Karatekin, B. , Icagasioglu, A. , Ulasoglu, C. , İçten, S. , & Incealtin, O. (2020). Clinical presentations of pain in patients with COVID‐19 infection. Irish Journal of Medical Science, 190(3), 913–917. Advance online publication. 10.1007/s11845-020-02433-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen, J. (2018). Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders. Cephalalgia, 38(1), 1–211. 10.1177/0333102417738202 [DOI] [PubMed] [Google Scholar]
- Pennisi, M. , Lanza, G. , Falzone, L. , Fisicaro, F. , Ferri, R. , & Bella, R. (2020). SARS‐CoV‐2 and the nervous system: From clinical features to molecular mechanisms. International Journal of Molecular Sciences, 21(15), 5475. 10.3390/ijms21155475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poncet‐Megemont, L. , Paris, P. , Tronchere, A. , Salazard, J. P. , Pereira, B. , Dallel, R. , Aumeran, C. , Beytout, J. , Jacomet, C. , Laurichesse, H. , Lesens, O. , Mrozek, N. , Vidal, M. , & Moisset, X. (2020). High prevalence of headaches during Covid‐19 infection: A retrospective cohort study. Headache, 60(10), 2578–2582. 10.1111/head.13923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah, S. , Danda, D. , Kavadichanda, C. , Das, S. , Adarsh, M. B. , & Negi, V. S. (2020). Autoimmune and rheumatic musculoskeletal diseases as a consequence of SARS‐CoV‐2 infection and its treatment. Rheumatology International, 40(10), 1539–1554. 10.1007/s00296-020-04639-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares, F. , Kubota, G. T. , Fernandes, A. M. , Hojo, B. , Couras, C. , Costa, B. V. , Lapa, J. , Braga, L. M. , Almeida, M. M. , Cunha, P. , Pereira, V. , Morais, A. , Teixeira, M. J. , Ciampi de Andrade, D. & “Pain in the Pandemic Initiative Collaborators” (2021). Prevalence and characteristics of new‐onset pain in COVID‐19 survivours, a controlled study. European Journal of Pain, 25(6), 1342–1354. 10.1002/ejp.1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, S. , Cui, H. , Wang, T. , Shen, X. , & Ma, C. (2020). Pain: A potential new label of COVID‐19. Brain, Behavior, and Immunity, 87, 159–160. 10.1016/j.bbi.2020.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uygun, Ö. , Ertaş, M. , Ekizoğlu, E. , Bolay, H. , Özge, A. , Kocasoy Orhan, E. , Çağatay, A. A. , & Baykan, B. (2020). Headache characteristics in COVID‐19 pandemic‐a survey study. The Journal of Headache and Pain, 21(1), 121. 10.1186/s10194-020-01188-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacchiano, V. , Riguzzi, P. , Volpi, L. , Tappatà, M. , Avoni, P. , Rizzo, G. , Guerra, L. , Zaccaroni, S. , Cortelli, P. , Michelucci, R. , & Liguori, R. (2020). Early neurological manifestations of hospitalized COVID‐19 patients. Neurological Sciences, 41(8), 2029–2031. 10.1007/s10072-020-04525-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hehn, C. A. , Baron, R. , & Woolf, C. J. (2012). Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron, 73(4), 638–652. 10.1016/j.neuron.2012.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng, L. M. , Su, X. , & Wang, X. Q. (2021). Pain symptoms in patients with coronavirus disease (COVID‐19): A literature review. Journal of Pain Research, 14, 147–159. 10.2147/JPR.S269206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widyadharma, I. , Dewi, P. R. , Wijayanti, I. , & Utami, D. (2020). Pain related viral infections: A literature review. The Egyptian Journal of Neurology, Psychiatry and Neurosurgery, 56(1), 105. 10.1186/s41983-020-00238-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Y. , Xu, X. , Chen, Z. , Duan, J. , Hashimoto, K. , Yang, L. , Liu, C. , & Yang, C. (2020). Nervous system involvement after infection with COVID‐19 and other coronaviruses. Brain, Behavior, and Immunity, 87, 18–22. 10.1016/j.bbi.2020.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W. , Du, R. H. , Li, B. , Zheng, X. S. , Yang, X. L. , Hu, B. , Wang, Y. Y. , Xiao, G. F. , Yan, B. , Shi, Z. L. , & Zhou, P. (2020). Molecular and serological investigation of 2019‐nCoV infected patients: Implication of multiple shedding routes. Emerging Microbes & Infections, 9(1), 386–389. 10.1080/22221751.2020.1729071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler, C. G. K. , Allon, S. J. , Nyquist, S. K. , Mbano, I. M. , Miao, V. N. , Tzouanas, C. N. , Cao, Y. , Yousif, A. S. , Bals, J. , Hauser, B. M. , Feldman, J. , Muus, C. , Wadsworth, M. H. , Kazer, S. W. , Hughes, T. K. , Doran, B. , Gatter, G. J. , Vukovic, M. , Taliaferro, F. , … Zhang, K. (2020). SARS‐CoV‐2 receptor ACE2 is an interferon‐stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell, 181(5), 1016–1035.e19. 10.1016/j.cell.2020.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Table S1‐S2
Supplementary Material


