Abstract
Introduction
Health professions are heavily engaged facing the current threat of SARS‐CoV‐2 (COVID‐19). Although there are many diagnostic tools, an accurate and rapid laboratory procedure for diagnosing COVID‐19 is recommended. We focused on platelet parameters as the additional biomarkers for clinical diagnosis in patients presenting to the emergency department (ED).
Materials and Methods
Five hundred and sixty‐one patients from February to April 2020 have been recruited. Patients were divided into three groups: (N = 50) COVID‐19 positive and (N = 21) COVID‐19 negative with molecular testing, (N = 490) as reference population without molecular testing. A Multiplex rRT‐PCR from samples collected by nasopharyngeal swabs was performed and the hematological data collected.
Results
We detected a mild anemia in COVID‐19 group and lymphopenia against reference population: hemoglobin (g/dL) 13.0 (11.5‐14.8) versus 13.9 (12.8‐15.0) (P = .0135); lymphocytes (109/L) 1.24 (0.94‐1.73) versus 1.99 (1.49‐2.64) (P < .0001). In addition, abnormal platelet parameters as follows (COVID group vs reference population): PLT (×109/L) 209 (160‐258) vs 236 (193‐279) (P = .0239). IPF (%) 4.05 (2.5‐5.9) versus 3.4 (2.2‐4.9) (P = .0576); H‐IPF (%) 1.25 (0.8‐2.2) versus 0.95 (0.6‐1.5) (P = .0171) were identified. In particular, COVID positive group had a high H‐IPF/IPF Ratio compared to reference population [0.32 (0.29‐0.36) versus 0.29 (0.26‐0.32), respectively, (P = .0003)]. Finally, a PLT difference of nearly 50 × 109/L between pre/postCOVID‐19 sampling for each patient was found (N = 42) (P = .0194).
Conclusions
COVID‐19 group results highlighted higher IPF and H‐IPF values, with increased H‐IPF/IPF Ratio, associated to PLT count reduction. These findings shall be adopted for a timely diagnosis of patients upon hospital admission.
Keywords: COVID‐19, emergency department, hematological biomarkers, H‐IPF, platelet parameters
1. INTRODUCTION
Since the beginning of 2020, the new viral infection called coronavirus disease 2019 (COVID‐19) has been putting our society to the test. Consequently, heathcare systems worldwide are heavily engaged to tackle infection‐related complications. In an emergency department (ED), timely diagnosis and treatment are required for patients seen for different diseases and in our cardiology ED, an increase of COVID‐19 suspected cases has resulted in an overcrowding of patients. This caused difficulties in triaging patients for rapid and accurate diagnosis and therapy. A large number of studies describing hematological and hemostatic alterations after the onset of COVID‐19 symptoms confirm the sequelae of multiorgan lesions of virus infection. Furthermore, most of the patients have mild symptoms such as fever, dry cough, dyspnea, myalgia, and overlapping symptoms with acute myocardial infarction (AMI) as discomfort and chest pain. Thus, it is necessary to quickly identify the infection using a real time reverse transcription polymerase chain reaction (rRT‐PCR) and/or rapid test with an integration of clinical and laboratory data. Application of algorithms lowering pressure on isolation rooms and to reduce the number of patients undergo to rRT‐PCR testing has been recently proposed. 1 , 2 , 3 , 4
Interestingly, the reduction of blood cells as lymphopenia and thrombocytopenia were well associated with COVID‐19 and its severity, 5 , 6 , 7 , 8 , 9 and studies on their use for the diagnosis are still ongoing. In particular, a low platelet count at hospital admission has been described as an independent risk factor for COVID‐19 disease progression and for in‐hospital mortality, 7 , 10 , 11 suggesting its potential use in this clinical setting. However, evidence for its diagnostic application as clinical biomarker is so far not established.
In this scenario, we investigated the hematological parameters, available in routine testing panel, upon cardiology ED admission, focusing on platelet parameters as potential additional biomarkers for COVID‐19 diagnosis.
2. MATERIALS AND METHODS
2.1. Study cohort and protocol
A retrospective and observational pilot study was conducted at the Centro Cardiologico Monzino, Milan, Italy, using the laboratory database and the automated hematology analyzer Sysmex XN (Sysmex corporation) software.
Five hundred and sixty‐one consecutive patients admitted to our Hospital with complete molecular and hematological data within the period February 1‐April 19, 2020 were included in the study.
An expert team of cardiologists garnered clinical diagnosis regarding predominantly as first symptom a pulmonary distress along with the suspicion of cardiological diseases. Hematological parameters were evaluated through a comparison between the COVID‐19 positive population (N = 50) and a reference population of patients presented to ED in the same period (N = 490). A specific comparison was made against a COVID‐19 negative population (N = 21). The study was approved by the Ethical Committee of our center, and conformed to the principles outlined in the Declaration of Helsinki.
2.2. Blood collection and measurement of hematological parameters
The analytical platform enabled blood cell counts using fluorescent dyes and specific analytical channels, particularly for fluorescent platelet count. Blood samples were drawn into anticoagulant K3 EDTA tube (Vacutainer BD, PL6 7BP, UK) and within 1 hour processed along with biochemical testing of routine triage panel.
Platelet count was performed in fluorescent flow cytometry with Limit of Detection (LOD) and Limit of Quantitation (LOQ) equal to 1 × 109/L. The immature platelet fraction (IPF) (reticulated platelets) and the highly fluorescence immature platelet fraction (H‐IPF) were measured, while the H‐IPF/IPF Ratio was calculated. The latter parameter is a new derived‐parameter introduced to gain more information on the platelet turnover and the hematopoietic activity in COVID‐19 patients. Basal count (preCOVID‐19 diagnosis) and postCOVID‐19 at the time of diagnosis for each patient have been evaluated in order to verify the PLT count reduction during viral disease (Figure 1).
FIGURE 1.
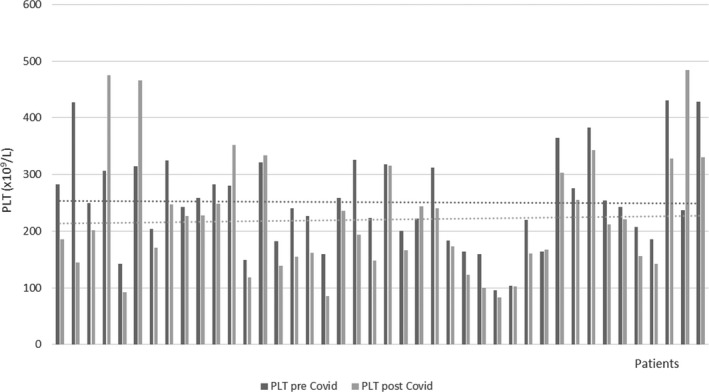
PLT count pre‐ and postCOVID‐19 diagnosis. Horizontal lines represent the graphical tendency of the PLT counts illustrating the reduced PLT count post infection
2.3. Multiplex rRT‐PCR assay
Molecular assays were carried out using a Multiplex rRT‐PCR from samples collected by nasopharyngeal swabs. Three viral genes: E, RdRP, N, to detect SARS‐CoV‐2 using GeneFinder COVID‐19 Plus RealAmp Kit (OSANG Healthcare, Anyangcheondong‐ro, Dongan‐gu, Anyang‐si, Gyeonggi‐do, Korea) on ELITech InGenius platform have been analyzed.
2.4. Statistical analysis
Statistical analysis was performed using SAS version 9.4 (SAS Institute). Continuous variables are presented as mean ± standard deviation (SD) and were compared using the t test for independent samples. Variables not normally distributed are presented as median and interquartile ranges and were compared with the Wilcoxon rank‐sum test. All performed analyses were adjusted for age and sex by general linear models. A P ≤ .05 was considered to be statistically significant.
3. RESULTS
3.1. Patients characteristics
Among 561 patients enrolled in this study, 50 were COVID‐19 positive, 21 COVID‐19 negative and 490 (reference population) without molecular testing and clinical signs of COVID‐19 infection, admitted to our ED as patients with the suspicion of cardiovascular diseases.
Clinical data of COVID‐19 positive patients displayed 38/50 (76%) of patients with SARS‐CoV‐2 pneumonia (Table 1) of which 31/38 (81.5%) with secondary diseases: (pneumococcal, enterococcus, HCV) superinfections, lower limb ischemia, myocarditis, rhythm disturbances, chronic obstructive pulmonary disease and emphysema, aortic dysfunction, pulmonary embolism and acute kidney injury (AKI). We recorded three severe SARS‐CoV‐2 pneumonia with major complications that ended in death.
TABLE 1.
Main clinical characteristics of patients presented at ED
| Primary Illness | SARS‐CoV‐2 positive test (N) | SARS‐CoV‐2 negative test (N) | Tot |
|---|---|---|---|
| ACS | 1 | 1 | 2 |
| AMI | 4 | 2 | 6 |
| SARS‐CoV‐2 pneumonia | 38 | 0 | 38 |
| Chest pain | 0 | 4 | 4 |
| Angina pectoris | 0 | 3 | 3 |
| SARS‐CoV‐2 infection without pneumonia | 7 | 0 | 7 |
| Respiratory insufficiency | 0 | 2 | 2 |
| HF and/or AF | 0 | 5 | 5 |
| Upper airways infection | 0 | 1 | 1 |
| Bronchiolitis | 0 | 1 | 1 |
| Aortic and/or mitral valve dysfunction | 0 | 1 | 1 |
| Interstitial pneumonia No SARS‐CoV‐2 | 0 | 1 | 1 |
| Total cases | 50 | 21 | 71 |
Abbreviations: ACS, acute coronary syndrome; AF, atrial fibrillation; AMI, acute myocardial infarction; HF, heart failure.
3.2. Hematological parameters in COVID‐19 positive patients compared to ED population or COVID‐19 negative group
Main hematological parameters are reported in Table 2. Hemoglobin concentration, erythrocytes, lymphocytes, eosinophils and basophils were lower in the COVID‐19 group compared to reference population of patients presented to ED (Table 2).
TABLE 2.
Main demographic and hematological data between COVID‐19 positive patients and a reference (ED) population
| COVID‐19 positive (n = 50) | ED population (n = 490) | P‐value | P‐value* | |||
|---|---|---|---|---|---|---|
| Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | |||
| Age (years) | 70.6 ± 12 | 61.2 ± 16.3 | <.001 | / | ||
| Male sex (%) | 66 | 59 | .349 | / | ||
| WBC (×109/L) | 8.6 ± 4.1 | 7.9 (5.8‐10.7) | 8.6 ± 2.7 | 8.1 (6.7‐9.9) | .2605 | / |
| RBC (×1012/L) | 4.4 ± 0.67 | 4.5 (3.9‐4.9) | 4.7 ± 0.6 | 4.7 (4.4‐5.1) | .0007 | .0055 |
| HGB (g/dL) | 13.1 ± 2.2 | 13(11.5‐14.8) | 13.8 ± 1.7 | 13.9 (12.8‐15.0) | .0135 | .0269 |
| HCT (%) | 39.5 ± 6.3 | 39.3 (35.2‐43.5) | 41.2 ± 4.6 | 41.7 (38.7‐44.2) | .0123 | .028 |
| MCV (fL) | 88.6 ± 5.2 | 89.4 (85.3‐91.5) | 86.7 ± 6.4 | 87.5 (84.5‐90.1) | .0401 | .3186 |
| MCH (pg) | 29.5 ± 2.1 | 29.9 (27.9‐30.8) | 29.0 ± 2.6 | 29.5 (28.3‐30.5) | .3967 | / |
| MCHC (g/L) | 323 ± 14 | 332 (323‐341) | 334 ± 12 | 336 (328‐343) | .1858 | / |
| RDW‐SD (fL) | 45.2 ± 6.4 | 44.3 (40.6‐47.6) | 42.8 ± 5.1 | 42.1 (39.5‐44.8) | .0062 | .1056 |
| RDW‐CV (%) | 14.0 ± 1.8 | 13.5 (12.8‐14.8) | 13.6 ± 1.7 | 13.2 (12.6‐14) | .0575 | / |
| NEUT (×109/L) | 6.3 ± 3.5 | 5.68 (3.6‐8.5) | 5.6 ± 2.4 | 5.1 (4.0‐6.7) | .4685 | / |
| LYMPH (×109/L) | 1.4 ± 1.1 | 1.24 (0.94‐1.73) | 2.1 ± 0.9 | 1.99 (1.49‐2.64) | <.0001 | .0004 |
| MONO (×109/L) | 0.67 ± 0.34 | 0.57 (0.46‐0.78) | 0.68 ± 0.33 | 0.63 (0.51‐0.77) | .3594 | / |
| EO (×109/L) | 0.06 ± 0.07 | 0.03 (0.01‐0.12) | 0.14 ± 0.14 | 0.1 (0.05‐0.19) | <.0001 | .0004 |
| BASO (×109/L) | 0.03 ± 0.02 | 0.03 (0.01‐0.04) | 0.04 ± 0.02 | 0.04 (0.03‐0.05) | <.0001 | .0029 |
Abbreviations: BASO, basophils; EO, eosinophils; HCT, haematocrit; HGB, hemoglobin; LYMPH, lymphocytes; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscolar hemoglobin concentration; MCV, mean corpuscular volume; MONO, monocytes; NEUT, neutrophils; RBC, red blood cells; RDW‐CV, red blood cell distribution width‐coefficient of variation; RDW‐SD, red blood cell distribution width‐standard deviation; WBC, white blood cells.
* P‐value obtained from the adjustment through age and sex.
When PLT parameters were analyzed in COVID‐19 positive ED population and COVID‐19 negative groups, we found that COVID‐19 positive group displayed a significant lower PLT count than the other two groups (Table 3 and 4). In addition, COVID‐19 positive patients have increased H‐IPF and IPF compared to ED population (Table 3) and to COVID‐19 negative group (Table 4), respectively. However, all these differences were lost after adjustment for age and sex.
TABLE 3.
Platelet parameters between COVID‐19 positive patients and a reference (ED) population
| COVID‐19 positive (n = 50) | ED population (n = 490) | P‐value | P‐value* | |||
|---|---|---|---|---|---|---|
| Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | |||
| PLT (×109/L) | 220 ± 85.5 | 209 (160‐258) | 241 ± 72.5 | 236 (193‐279) | .0239 | .2998 |
| PDW (fL) | 12.6 ± 2.2 | 12.2 (11.1‐14.1) | 12.4 ± 1.98 | 12.1 (10.6‐13.6) | .5258 | / |
| MPV (fL) | 10.5 ± 1.0 | 10.4 (10‐11.3) | 10.4 ± 0.9 | 10.4 (9.8‐11) | .2584 | / |
| P‐LCR (%) | 29.6 ± 8.1 | 28.9 (24.6‐35.4) | 28.8 ± 7.2 | 28.2 (23.6‐33.7) | .3519 | / |
| PCT (%) | 0.23 ± 0.08 | 0.24 (0.18‐0.29) | 0.25 ± 0.07 | 0.25 (0.21‐0.29) | .0443 | .2218 |
| H‐IPF (%) | 1.71 ± 1.6 | 1.25 (0.8‐2.2) | 1.27 ± 1.2 | 0.95 (0.6‐1.5) | .0171 | .1247 |
| IPF (×109/L) | 9.4 ± 5.4 | 7.45 (5.9‐11.6) | 8.5 ± 4.7 | 7.9 (5.3‐10.5) | .3622 | / |
| IPF (%) | 4.7 ± 3.3 | 4.05 (2.5‐5.9) | 3.9 ± 2.6 | 3.4 (2.2‐4.9) | .0576 | / |
| Ratio (H‐IPF/IPF) | 0.33 ± 0.06 | 0.32 (0.29‐0.36) | 0.29 ± 0.05 | 0.29 (0.26‐0.32) | .0003 | .0102 |
Abbreviations: H‐IPF, high‐fluorescent immature platelet fraction; IPF, immature platelet fraction; MPV, mean platelet volume; PCT, plateletcrit; PDW, platelet distribution width; P‐LCR, platelet‐large cell ratio; PLT, platelets.
*P‐value obtained from the adjustment through age and sex.
TABLE 4.
Platelet parameters between COVID‐19 positive and COVID‐19 negative patients
| COVID‐19 positive (n = 50) | COVID‐19 negative (n = 21) | P‐value | P‐value* | |||
|---|---|---|---|---|---|---|
| Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | |||
| PLT (×109/L) | 220 ± 85.5 | 209 (160‐258) | 258 ± 78.9 | 248 (209‐268) | .0338 | .1587 |
| PDW (fL) | 12.6 ± 2.2 | 12.2 (11.1‐14.1) | 11.8 ± 1.8 | 11.6 (10.2‐13.1) | .1547 | / |
| MPV (fL) | 10.5 ± 1.0 | 10.4 (10‐11.3) | 10.2 ± 0.8 | 10.1 (9.5‐11) | .1074 | / |
| P‐LCR (%) | 29.6 ± 8.1 | 28.9 (24.6‐35.4) | 27.2 ± 7.2 | 26.2 (20.5‐33.4) | .1511 | / |
| PCT (%) | 0.23 ± 0.08 | 0.24 (0.18‐0.29) | 0.26 ± 0.08 | 0.24 (0.22‐0.31) | .1434 | / |
| H‐IPF (%) | 1.71 ± 1.6 | 1.25 (0.8‐2.2) | 1.03 ± 0.6 | 0.8 (0.5‐1.35) | .0523 | / |
| IPF (×109/L) | 9.4 ± 5.4 | 7.45 (5.9‐11.6) | 8.1 ± 4.11 | 7.4 (4.6‐10.35) | .3766 | / |
| IPF (%) | 4.7 ± 3.3 | 4.05 (2.5‐5.9) | 3.3 ± 1.6 | 3 (1.85‐4.5) | .0488 | .1525 |
| Ratio (H‐IPF/IPF) | 0.33 ± 0.06 | 0.32 (0.29‐0.36) | 0.30 ± 0.04 | 0.29 (0.27‐0.31) | .0935 | / |
Abbreviations: H‐IPF, high‐fluorescent immature platelet fraction; IPF, immature platelet fraction; MPV, mean platelet volume; PCT, plateletcrit; PDW, platelet distribution width; P‐LCR, platelet‐large cell ratio; PLT, platelets.
*P‐value obtained from the adjustment through age and sex.
Interestingly, the H‐IPF/IPF Ratio was higher in COVID‐19 positive group than in ED population, and after adjustment for confounder factors, this difference was maintained (Table 3). Derived platelet parameters including platelet distribution width (PDW), mean platelet volume (MPV), platelet‐large cell ratio (P‐LCR), as well as the absolute IPF count (IPF × 109/L), were similar between the groups (Table 3 and 4). In particular, absolute immature platelets did not reach a significant difference between groups likely due to platelet recovery that occurs after COVID‐19 infection.
3.3. Changes in consecutive PLT counts during viral infection
Analyzing the PLT behavior during viral infection, we observed a reduction in PLT count in patients with COVID‐19 (N = 42) (P = .0194), with a median difference value of nearly 50 × 109/L between preCOVID‐19 and postCOVID‐19 samples: 242.5 (188.8‐310.5 × 109/L) and 190 (145.7‐253.2 × 109/L), respectively, (Figure 1). This finding might suggest a platelet involvement even in the early stages of viral infection. However, four cases of increased PLT counts postCOVID‐19 disease due to two pneumococcal superinfections and two reactive thrombocytosis were found.
4. DISCUSSION
In a cardiology ED, a time consuming diagnostic procedure may affect therapeutic treatment. Therefore, patient triage in the COVID‐19 era represents a difficult problem for healthcare professions. In the first COVID‐19 outbreak, a detrimental reduction in hospital admissions for acute coronary syndrome (ACS) in northern Italy was observed, with a consequent increase in mortality. 12 For this reason, a rapid and efficient diagnostic strategy for the detection of SARS‐CoV‐2 may improve the treatment of COVID‐19 such as the management of cardiovascular diseases. Many studies have recently shown that COVID‐19 is not only a respiratory syndrome with endothelial and pulmonary impairments 13 but also a systemic pathology affecting many organs as well as the hematopoietic and hemostatic systems. 14 , 15 This implies that, in different phases of the COVID‐19 evolution, blood parameters may easily be adopted for the characterization of disease. Our results in line with many works 16 , 17 , 18 , 19 , 20 , 21 confirmed the alterations, even in the early phases, of hematological parameters 22 , 23 along with well‐known metabolic and biochemical derangements. In fact, we highlighted a mild anemia and thrombocytopenia in COVID‐19 patients, lymphopenia included in most cases a reduction in the total white blood cell count; as consequence, we obtained a low absolute count of eosinophils and basophils. Indeed, PLT count reduction, IPF and H‐IPF in the COVID‐19 patients were obtained by the comparison among the groups without age and sex adjustment (Table 3 and 4). This leads to interpreting our data specifically for a cardiology population due to the biological characteristics and influence of age and sex. Nonetheless, the H‐IPF/IPF Ratio seems increased in the COVID‐19 patients also after correction for the two confounder factors (Table 3). Of note, the features of a patient population should not affect this parameter, making it more suitable for different clinical settings. To our knowledge, the H‐IPF/IPF Ratio was first described, indicating larger platelets with very high fluorescence, therefore, more activated during the pathogenesis of infection. Longitudinal evaluation of patients showed a significant platelet reduction from preCOVID‐19 to postCOVID‐19 state suggesting that platelets may be the first responders in innate immunity along with leucocytes. As worthily described by the authors Mcfadyen and Goshua, the reduction of platelet count may be the sentinel of the viral infection that signals endotheliopathy, leukocytes activation and the thrombo‐inflammation state in COVID‐19 patients. 21 , 24 According to these outcomes, an early treatment of infected patients would be desirable, perhaps based on antithrombotic drugs, according to increasing evidence on the involvement of coagulation from the beginning of the viral infection. 25 , 26 Importantly, the pharmacological effects of anti‐inflammatory and antiplatelets drugs will be determined by controlled clinical trials currently underway. Our observations suggest a key role for platelets not only in the evolution but also in the early stages of the disease, therefore, useful for COVID‐19 diagnosis. However, we admit that our study has some limitations. As retrospective investigation, we considered the laboratory data of patients only at the time of ED admission diagnostically relevant. Furthermore, many results once adjusted for age and sex lose statistical significance, suggesting that our data should be confirmed by other studies with a larger number of patients. In conclusion, the platelet parameters could be useful for the classification of patients suspected of COVID‐19 upon hospital admission. For example, their evaluation could help the diagnostic interpretation of SARS‐CoV‐2 antigen 27 or molecular rapid tests, in terms of pretest probability of viral illness. Moreover, the hematological biomarkers together with biochemical and clinical information shall be applied for assessing the COVID‐19 risk using machine learning or diagnostic scoring procedures.
CONFLICT OF INTEREST
The authors have no competing interests.
AUTHOR CONTRIBUTIONS
AB analyzed data and involved in conceptualization of the work. GI designed, conducted, analyzed data, and wrote the manuscript. TD and AC conducted laboratory work. LS, EA collected clinical data. SSB conducted supervision and writing‐review. MLB involved in supervision.
ACKNOWLEDGEMENT
We thank all the people who contributed to the work during this difficult time. This study did not receive specific funding but was performed as part of the Italian Research Project n. R1312/‐CCM 1380.
Introcaso G, Bonomi A, Salvini L, et al. High immature platelet fraction with reduced platelet count on hospital admission. Can it be useful for COVID‐19 diagnosis? Int J Lab Hematol. 2021;43:1319–1324. 10.1111/ijlh.13701
REFERENCES
- 1. Kurstjens S, van der Horst A, Herpers R, et al. Rapid identification of SARS‐CoV‐2 infected patients at the emergency department using routine testing. Clin Chem Lab Med. 2020;58(9):1587‐1593. [DOI] [PubMed] [Google Scholar]
- 2. Lippi G, Henry BM, Hoehn J, Benoit S, Benoit J. Validation of the corona score for rapid identification of Sars Cov‐2 infections in patients seeking emergency department care in the United States. Clin Chem Lab Med. 2020;58(12):e311‐e313. 10.1515/cclm-2020-1121 [DOI] [PubMed] [Google Scholar]
- 3. Assandri R, Canetta C, Viganò G, Buscarini E, Cartabellati A, Montanelli A. Laboratory markers included in the corona score can identify false negative results on COVID‐19 RT‐PCR in the emergency room. Biochem Med. 2020;30:030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Assandri R, Montanelli A. Modified corona score can easily identify covid‐19 patients with gastrointestinal symptoms: an Italian proposal. Gastroenterol Hepatol Bed Bench. 2020;13(4):393‐395. [PMC free article] [PubMed] [Google Scholar]
- 5. Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease (COVID‐19) infections: a meta‐analysis. Clin Chim Acta. 2020;506:145‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zheng Y, Zhang Y, Chi H, et al. The hemocyte counts as a potential biomarker for predicting disease progression in COVID‐19: a retrospective study. Clin Chem Lab Med. 2020;58(7):1106‐1115. [DOI] [PubMed] [Google Scholar]
- 7. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Qu R, Ling Y, Zhang YHZ, et al. Platelet‐to lymphocyte ratio is associated with prognosis in patients with coronavirus disease‐19‐. J Med Virol. 2020;92(9):1533‐1541. 10.1002/jmv.25767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tachil J. What do monitoring platelet counts in COVID‐19 teach us? J Thromb Haemost. 2020;18(8):2071‐2072. 10.1111/jth.14879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bi X, Su Z, Yan H, et al. Prediction of severe illness due to COVID‐19 based on an analysis of initial fibrinogen to albumin ratio and platelet count. Platelets. 2020;31(5):674‐679. 10.1080/09537104.2020.1760230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu Y, Sun W, Guo Y, et al. Association between platelet parameters and mortality in coronavirus disease 2019: retrospective cohort study. Platelets. 2020;31(4):490‐496. 10.1080/09537104.2020.1754383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. De Filippo O, D’Ascenzo F, Angelini F, et al. Reduced rate of hospital admission for ACS during Covid‐19 outbreak in Northern Italy. N Engl J Med. 2020;383(1):88‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marongiu F, Grandone E, Barcellona D. Pulmonary thrombosis in 2019‐nCov Pneumonia? J Thromb Haemost. 2020;18(6):1511‐1513. 10.1111/jth.14818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lippi G, Plebani M. Cytokine ‘’storm’’, cytokine ‘’breeze’’, or both in Covid‐19? Clin Chem Lab Med. 2021;59(4):637‐639. 10.1515/cclm-2020-1761 [DOI] [PubMed] [Google Scholar]
- 15. Lippi G, Sanchis‐Gomar F, Henry BM. Covid‐19: unravelling the clinical progression of nature’s virtually perfect biological weapon. Ann Transl Med. 2020;8:693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Amgalan A, Othman M. Hemostatic laboratory derangement in Covid‐19 with a focus on platelet count. Platelets. 2020;31(6):740‐745. [DOI] [PubMed] [Google Scholar]
- 17. Soraya GV, Ulhaq ZS. Crucial laboratory parameters in Covid‐19 diagnosis and prognosis: an updated meta‐analysis. Med Clin (Barc). 2020;155(4):143‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sigaroodi AP, Bashash D, Fateh F, Abolghasemi H. Laboratory findings in Covid‐19 diagnosis and prognosis. Clin Chim Acta. 2020;510:475‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yuan X, Huang W, Ye B, et al. Changes of hematological and immunological parameters in COVID‐19 patients. Int J Hematol. 2020;112(4):553‐559. 10.1007/s12185-020-02930-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khandait H, Gandotra G, Sachdeva S, et al. Covid‐19 and hematology‐what do we know so far? SN Compr Clin Med. 2020;2:2631‐2636. 10.1007/s42399-20-00607-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goshua G, Pine AB, Meizlish ML, et al. Endoteliopathy in COVID‐19‐associated coagulopathy: evidence from a single‐centre, cross‐sectional study. Lancet Haematol. 2020;7:e575‐e582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lombardi A, Trombetta E, Cattaneo A, et al. Early phases of COVID‐19 are characterized by a reduction in lymphocyte populations and the presence of atypical monocytes. Front Immunol. 2020;11:560330. 10.3389/fimmu.2020.560330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Frater JL, Zini G, d’Onofrio G, Rogers HJ. COVID‐19 and the clinical hematology laboratory. Int J Lab Hematol. 2020;42(S1):11‐18. 10.1111/ijlh.13229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. MacFadyen JD, Stevens H, Peter K. The emerging threat of (Micro) thrombosis in COVID‐19 and its thearapeutic implications. Circ Res. 2020;127:571‐587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. White D, MacDonald S, Edwards T, et al. Evaluation of COVID‐19 coagulopathy; laboratory characterization using thrombin generation and nonconventional haemostasis assays. Int J Lab Hematol. 2021;43:123‐130. [DOI] [PubMed] [Google Scholar]
- 26. Nugroho J, Wardhana A, Maghfirah I, et al. Relationship of D‐dimer with severity and mortality in SARS‐CoV‐2 patients : a meta‐analysis. Int J Lab Hematol. 2021;43:110‐115. [DOI] [PubMed] [Google Scholar]
- 27. Mattiuzzi C, Henry BM, Lippi G. Making sense of rapid antigen testing in severe acute respiratory syndrome coronavirus 2 (SARS‐Cov‐2) diagnostics. Diagnosis. 2021;8(1):27‐31. 10.1515/dx-2020-0131 [DOI] [PubMed] [Google Scholar]


