Figure 4.
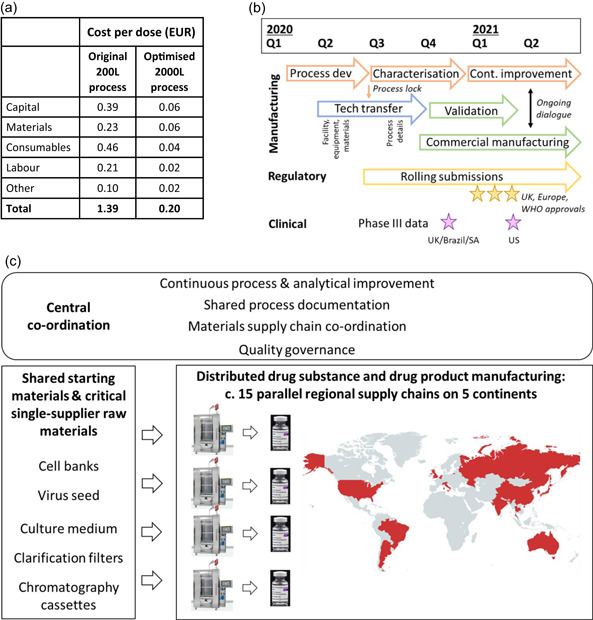
Rapid implementation of a low cost distributed manufacturing strategy. (a) tabulates modeled costs of bulk DS production using the initial process at 200 L scale (with high MOI and including the additional TFF step), and using the optimized process at 2000L scale (with low MOI and direct AEX). This excludes fill/finish and some analytical costs. For further details, please see Supporting Information. (b) A timeline of key manufacturing‐related activities, highlighting activities performed in parallel and relationship to the timing of key regulatory and clinical events. (c) illustrates global distributed manufacturing strategy, with interplay between centrally co‐ordinated activities, common origins of certain key materials, and multiple parallel regional drug substance and drug product supply chains. ChAdOx1 nCoV‐19 drug substance is currently being manufactured in the countries shown in red. Vial photograph: Arne Müseler/arne‐mueseler.com/CC‐BY‐SA‐3.0/https://creativecommons.org/licenses/by-sa/3.0/de/deed.de. Map created using mapchart.com, under CC‐BY‐SA‐4.0 licence. AEX, anion exchange; DS, drug substance; MOI, multiplicity of infection; TFF, tangential flow filtration
