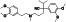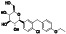TABLE 1.
Blocking virus‐cell membrane fusion and entry
| Drug name and structure | Original mechanism | FDA‐approved indication(s) | Possible mechanism for anti‐COVID‐19 | Clinical trials ID (stage) | The highest anti‐COVID‐19 phase/ID/status/start–(estimated)end dates | References |
|---|---|---|---|---|---|---|
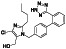
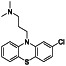
|
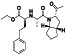
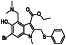
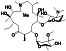
Ramipril
|
ACE inhibitor | Type 2 diabetes, vascular disease, hypertension, metabolic syndrome X, peripheral arterial disease, kidney transplant | ACE inhibitor | NCT04366050 (Phase 2) | Phase 2/NCT04366050/enrolling by invitation/May Huaier Granule 11, 2020–May 2021 | (Amat‐Santos et al., 2020) |
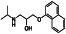
|
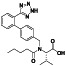
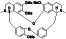
Atovaquone
|
Mitochondrial cytochrome bc1 complex inhibitor, antimalarial agent | HIV infections, malaria, plasmodium falciparum malaria, rabies | Elevating endosomal pH and interfere with ACE2 glycosylation |
NCT04339426 (Phase 2) NCT04456153 (Phase 2) |
Phase 2/NCT04456153/completed/July 22, 2020–January 31, 2021 | (Clinical trial ID: NCT04456153, n.d.; Farag et al., 2020; Sachdeva et al., 2020) |
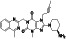
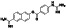
|
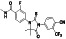
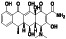
Enzalutamide
|
Androgen receptor (AR) antagonist | Prostate cancer | Blocking TMPRSS2 |
NCT04456049 (Phase 2) NCT04475601 (Phase 2) |
Phase 2/NCT04475601/recruiting/July 15, 2020–July 8, 2021 | (Cattrini et al., 2020) |
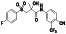
|
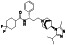
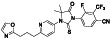
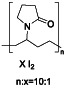
Maraviroc
|
CCR5 antagonist | HIV infection, endothelial dysfunction, | Inhibiting s‐protein mediated cell fusion and SARS‐CoV‐2 multiplication |
NCT04441385 (Phase 2) NCT04475991 (Phase 2) NCT04710199 (Phase 2) NCT04435522 (Phase 1) |
Phase 2/NCT04441385/recruiting/June 26, 2020–November 30, 2020 |
(Koenig et al., 2020; Risner et al., 2020; Shamsi et al., 2020) |
|
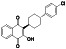
Losartan
|
AT II receptor antagonist | Hypertension, kidney disease, proteinuria, emphysema, colitis, etc. | ACE2 blocker preventing virus entry into the host cells |
Approximately 8 clinical trials, some of the examples are: NCT04335123 (Phase 1) NCT04447235 (Phase 2) NCT04428268 (Phase 2) NCT04643691 (Phase 2) NCT04311177 (Phase 2) NCT04312009 (Phase 2) NCT04606563 (Phase 3) NCT04343001 (Phase 3) |
Phase3/NCT04606563/recruiting/October 9, 2020–June 30, 2021 | |
|
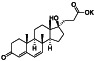
Isotretinoin (13‐cis‐retinoic acid)
|
Anti‐apoptosis | Severe acne | Down‐regulates ACE2 receptors, combination with all‐trans retinoic acid may enhances neutralizing antibodies in COVID‐19 infected Patients |
Approximately 9 clinical trials, some of the examples are: NCT04353180 (Phase 3) NCT04396067 (Phase 2) NCT04577378 (Phase 2) NCT04389580 (Phase 2) NCT04578236 (Phase 2) NCT04361422 (Phase 3) NCT04382950 (Phase 1) |
Phase 3/NCT04353180/not yet recruiting/October 2020–June 2021 |
(Hamouda Elgarhy, 2020) |
|
Lactoferrin (protein) |
Anti‐microbial, anti‐inflammatory, and immunomodulatory |
Anti‐gram‐negative, anti‐gram‐positive bacteria, fungi and viruses |
Inhibiting the union of ACE2 with SARS‐CoV‐2 spike protein, blocking the heparan sulfate proteoglycan receptor |
NCT04526821 (Phase 2) NCT04421534 (Phase 3) NCT04412395 (Phase 3) NCT04427865 (Phase 3) NCT04847791(not applicable) NCT04475120 (Phase 3) NCT04713735 (not applicable) |
Phase 3/NCT04475120/completed/April 15, 2020–July 2, 2020 | |
|
Propranolol
|
Non‐selective β1/β2‐blocker |
Hyperalgesia, social anxiety disorder, migraine headache, cigarette smoking, asthma, etc. | Blocking entry of SARS‐COV‐2 through downregulation of the ACE2 receptor and CD147 | NCT04467086 (Phase 3) | Phase 3/NCT04467086/not yet recruiting/December 2020–April 2021 |
(Vasanthakumar, 2020) |
|
Linagliptin
|
DPP‐4 inhibitor | Diabetes mellitus, type 2 | Inhibiting DPP4, blocking DPP4 inteacting with spike protein of the MERS‐Co‐V | NCT04371978 (Phase 3) | Phase 3/NCT04371978/recruiting/October 1, 2020–June 30, 2021 |
(Solerte et al., 2020) |
|
Verapamil
|
Calcium channel blocker and P‐gp inhibitor, CYP3A4 inhibitor | Heart arrhythmias, high blood pressure and angina research |
A slow‐channel calcium‐blocking agent. Blocking ion channels to inhibit coronavirus entry. |
NCT04351763 (Phase 3) |
Phase 3/NCT04351763/recruiting/April 27, 2020–March 2, 2021 |
(Clinical trial ID: NCT04351763, n.d.) |
|
Chlorpromazine
|
Antagonist of dopamine D2, 5‐HT2A, potassium channel and sodium channel. | Psychotic disorders such as schizophrenia. Glioblastoma multiforme | K+/Na+ channel inhibitor, inhibits clathrin‐mediated endocytosis, |
NCT04366739 (Phase 3) NCT04354805 (Phase 3) |
Phase 3/NCT04366739/not yet recruiting/April 29, 2020–August 30, 2020 |
(Plaze et al., 2020) |
|
Dapagliflozin
|
Competitive SGLT2 inhibitor | Diabetes mellitus (DM) | Reducing the viral load and preventing the lowering of cytosolic pH | NCT04350593 (Phase 3) | Phase 3/NCT04350593/active, not recruiting/April 22, 2020–April 2021 |
(Cure & Cumhur Cure, 2020) |
|
Nafamostat
|
Serine protease inhibitor, | Acute kidney injury | TMPRSS2 inhibitor |
NCT04390594 (Phase 3) NCT04352400 (Phase 3) NCT04418128 (Phase 3) |
Phase 3/NCT04390594/recruiting/August 13, 2020–February 12, 2021 |
(Fernandez‐Fernandez et al., 2020) |
|
Bicalutamide
|
Non‐steroidal AR antagonist | Prostate cancer, neoplasms, prostate, | Blocking TMPRSS2 |
NCT04509999 (Phase 3) NCT04652765 (Phase 1) |
Phase 3/NCT04509999/recruiting/October 26, 2020–September 2022 |
(Cattrini et al., 2020) |
|
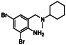
Bromhexine
|
Clearing mucus from respiratory tract, antioxidant | Chronic bronchitis, asthma and other causes of phlegm not easy to cough out | TMPRSS2 inhibitor |
NCT04340349 (Early Phase1) NCT04424134 (Phase 3) NCT04355026 (Phase 4) NCT04273763 (not applicable) |
Phase 4/NCT04355026/recruiting/April 10, 2020–June 30, 2020 | |
|
Camostat
|
Serine protease inhibitor, | Corona virus infection, COVID‐19, coagulopathy cardiovascular complication COVID‐19 | TMPRSS2 inhibitor |
Approximately 19clinical trials, some of the examples are: NCT04353284 (Phase 2) NCT04524663 (Phase 2) NCT04583592 (Phase 2) NCT04608266 (Phase 3) NCT04455815 (Phase 3) NCT04657497 (Phase 3) NCT04530617 (Phase 2) |
Phase 4/NCT04338906/withdrawn (lack of public funding)/June 1, 2020–December 2021 |
(Huang et al., 2020) |
|
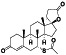
Spironolactone
|
AR antagonist | Heart failure, cardiomyopathy, alcoholic, alcoholism, osteoarthritis, Hypoglycemia, healthy | Inhibiting the androgen‐dependent expression of TMPRSS2 |
NCT04643691 (Phase 2) NCT04424134 (Phase 3) NCT04826822 (Phase 3) NCT04345887 (Phase 4) |
Phase 4/NCT04345887/not yet recruiting/April 21, 2020–July 21, 2020 | |
|
Valsartan (diovan)
|
AT II receptor antagonist | Hypertension stage 2 systolic hypertension |
ACE2 inhibitor, inhibition of TMPRSS2 |
NCT04335786 (Phase 4) | Phase 4/NCT04335786/recruiting/April 17, 2020–December 2020 |
(Vitiello et al., 2020) |
|
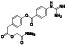
Arbidol (umifenovir)
|
Antiviral agent | Influenza |
Inhibition of ACE2/S protein interaction, blocking membrane fusion |
NCT04350684 (Phase 4) NCT04260594 (Phase 4) NCT04476719 (Phase 1) |
Phase 4/NCT04350684/enrolling by invitation/April 15, 2020–April 22, 2020 |
(Clinical trial ID: NCT04260594, n.d.; Clinical trial ID: NCT04252885, n.d.; Huang et al., 2020) |
|
Proxalutamide (GT0918)
|
Androgen receptor (AR) antagonist | COVID‐19, Prostate cancer |
ACE2 and TMPRSS2 levels in lung and cardiac cells are reduced by ant androgens |
NCT04853134 (Phase 3) NCT04728802 (Phase 3) NCT04853927 (Phase 3) NCT05009732 (Phase 3) NCT04870606 (Phase 3) NCT04446429 (not applicable) |
Phase 3/NCT04728802/completed/January 28, 2021–April 15, 2021 | (McCoy et al., 2021) |
|
Canrenoate potassium
|
Competitive mineralocorticoid receptor (aldosterone receptor) antagonist | Brain‐dead organ donors, Cirrhosis, COVID‐19 pneumonia |
Pleiotropic effects with favorable renin–angiotensin–aldosterone system (RAAS) and ACE2 expression, reduction in transmembrane serine protease 2 (TMPRSS2) activity and antiandrogenic action |
NCT04977960 (Phase 2) NCT04912011 (Phase 4) |
Phase 4/NCT04912011/recruiting/June 3, 2021–August 31, 2021 | (Kotfis & Lechowicz, 2021:) |
|
Defibrotide
|
Thrombotic microangiopathies, Kawasaki disease, Sickle cell disease | Coagulopathy | Mediated by the p38 MAPK pathway, which is upregulated as a result of the binding of SARSCoV2 on ACE2 receptors on the surface of endothelial cells and, in turn, activates the transcription of the proinflammatory cytokines. |
NCT04335201 (Phase 2) NCT04652115 (Phase 2) NCT04530604 (Ohase 1) NCT04348383 (Phase 2) |
Phase 2/NCT04335201/recruiting/April 6, 2020–June 20, 2021 |
(Macciò et al., 2020) |
|
Azithromycin
|
Inhibit translation via interacting with 50S subunit of the bacterial ribosome | Acute bacterial, community‐acquired pneumonia, uncomplicated skin infections, et al |
Inhibiting viral invasion via interact with spike protein/CD147 interaction or blocking CD147 expression |
Approximately 52 clinical trials, some of the examples are: NCT04334382 (Phase 3) NCT04371406 (Phase 3) NCT04359316 (Phase 4) NCT04339816 (Phase 3) NCT04332107 (Phase 3) NCT04621461 (Phase 4) NCT03871491 (Phase 3) |
Phase 4/NCT04621461/completed/November 2020–February 8, 2021 | |
|
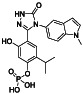
Tetrandrine
|
Voltage‐gated Ca2+ current (ICa) and Ca2+‐activated K+ current inhibitor | Corona virus disease 2019, COVID‐19 | Inhibiting voltage‐gated Ca2+ current (ICa) and Ca2+‐activated K+ current. | NCT04308317 (Phase 4) | Phase 4/NCT04308317/enrolling by invitation/March 5, 2020–March 1, 2021 |
(Clinical trial ID: NCT04308317, n.d.; Heister & Poston, 2020) |
|
Doxycycline
|
Broad‐spectrum metalloproteinase (MMP) inhibitor | Anal chlamydia infection, rosacea, overactive bladder, HIV infections, sinusitis, acne vulgaris | Inhibition of the E2 envelope glycoprotein involved in virus entry |
Approximately 10 clinical trials, some of the examples are: NCT04591600 (Phase 2) NCT04523831 (Phase 3) NCT04371952 (Phase 3) NCT04370782 (Phase 4) NCT04407130 (Phase 2) NCT04551755 (Phase 2) NCT04584567 (Phase 3) |
Phase 4/NCT04370782/completed/28, 2020–September 30, 2020 | |
|
Povidone‐iodine
|
Antibacterial agent (MRSA and MSSA strains) | External uses‐infection; cesarean section, vaginal surgeries, arrhythmia |
Interacting with surface proteins of enveloped viruses, destabilize membrane fatty acids, inducing cell apoptosis |
Approximately 12clinical trials, some of the examples are: NCT04410159 (Phase 2) NCT04549376 (Phase 2) NCT04510402 (Phase 2) NCT04371965 (Phase 2) NCT04872686 (Phase 3) NCT04344236 (Phase 2) NCT04603794 (Phase 4) |
Phase 4/NCT04603794/recruiting/October 1, 2020–November 2020 |
(Pattanshetty et al., 2021) |