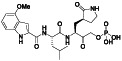
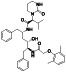
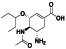
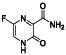
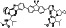
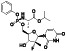
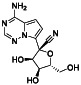
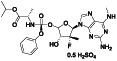
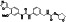TABLE 2.
Inhibiting virus replication
| Drug | Original mechanism | FDA‐approved indication(s) | Possible mechanism for anti‐COVID‐19 | Clinical trials ID (stage) | The highest anti‐COVID‐19 phase/ID/status/start–(estimated)end dates | References |
|---|---|---|---|---|---|---|
|
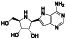
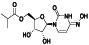
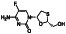
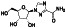
Galidesivir
|
Viral RNA‐dependent RNA polymerase (RdRP) inhibitor | Not yet approved | Inhibiting RNA dependent RNA polymerase | NCT03891420 (Phase 1) | Phase 1/NCT03891420/recruiting/April 9, 2020–May 31, 2021 | |
|
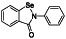
Ebselen (PZ‐51, SPI‐1005, CCG‐39161,)
|
Potent voltage‐dependent calcium channel (VDCC) blocker | Not yet approved | Inhibiting main protease via binding to Mpro from forming selenosulfide |
NCT04484025 (Phase 2) NCT04483973 (Phase 2) |
Phase 2/NCT04484025/not yet recruiting/ May 2021–June 2022 |
|
|
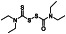
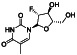
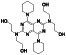
Disulfiram (tetraethylthiuram disulfide; TETD)
|
Aldehyde‐dehydrogenase (ALDH1) inhibitor | Cocaine addictive, chronic alcoholism |
Blocking the Mpro protease, inhibiting cytokine release induced by NF‐kB and NLRP inflammasome |
NCT04485130 (Phase 2) NCT04594343 (Phase 2) | Phase 2/NCT04594343/recruiting/November 20, 2020 –July 20, 2021 | |
|
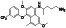
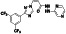
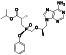
Tafenoquine
|
Anti‐malarial prophylactic agent. | Not yet approved | Inhibiting virus infection via down regulating Mpro activity | NCT04533347 (Phase 2) | Phase 2/NCT04533347/recruiting/February 19, 2021–June 8, 2021 | |
|
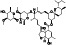
EIDD‐2801 (Molnupiravir, MK‐4482)
|
Virus RNA mutation | Antivirus, not yet approved | Induced RNA mutagenesis by the viral RNA‐dependent RNA polymerase (RdRp) | NCT04405739 (Phase 2) NCT04392219 (Phase 1) NCT04575584 (Phase 3) NCT04405570 (Phase 2) | Phase 3/NCT04575584/active, not recruiting/October 5, 2020–August 10, 2021 | (Sheahan, Sims, Zhou, et al., 2020; PubChem, National Library of Medicine, n.d.; Kabinger & Stiller, 2021) |
|
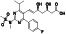
Merimepodib(MMPD, VX‐497)
|
Noncompetitive inosine monophosphate dehydrogenase (IMPDH) inhibitor | Not yet approved | Inhibiting Zika viral RNA replication | NCT04410354 (Phase 2) | Phase 2/NCT04410354/terminated (Failure to meet primary endpoint)/June 16, 2020–December 1, 2020 |
(X. Tong et al., 2018) |
|
Clevudine (L‐FMAU)
|
Nucleoside analog of the unnatural L‐configuration, non‐competitive inhibitor that is not incorporated into the viral DNA but rather binds to the polymerase. | Chronic hepatitis B | Inhibition of viral RNA synthesis |
NCT04891302 (Phase 2) NCT04347915 (Phase 2) |
Phase 2/NCT04347915/recruiting/April 15, 2020–January 30, 2021 | (Hui & Lau, 2005; Jang et al., 2011) |
|
Selinexor
|
Selective nuclear transport (SINE) inhibitor | Multiple myeloma | Interference nuclear export of vRNPs, viral mRNAs mediated by XPO1, blocking late‐stage viral assembly processes | NCT04349098(Phase 2) NCT04355676(Phase 2) | Phase 2/NCT04349098/completed/April 17, 2020–October 5, 2020 |
(Uddin et al., 2020) |
|
Emtricitabine
|
Nucleoside reverse transcriptase inhibitor (NRTI). | HIV infection, PrEP adherence monitoring, healthy, hepatitis B | Reverse transcriptase inhibitor |
NCT04712357 (not applicable) NCT04519125 (Phase 2) NCT04334928 (Phase 3) NCT04405271 (Phase 3) |
Phase 3/NCT04405271/recruiting/July 31, 2020–April 1, 2021 |
(Ayerdi et al., 2020) |
|
Tenofovir alafenamide
|
HIV‐1 nucleotide reverse transcriptase inhibitor | HIV infections, hepatitis B. | Reverse transcriptase inhibitor |
NCT04519125 (Phase NCT04334928 (Phase 3) NCT04405271 (Phase 3) |
Phase 3/NCT04405271/not yet recruiting/July 31, 2020–November 15, 2020 |
(Duan et al., 2020) |
|
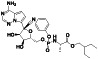
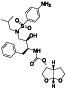
Remdesivir
|
Nucleoside analog with effective antiviral activity | Not yet approved |
Viral RNA‐dependent RNA polymerase (RdRP) inhibitor, reducing viral multiplication |
Approximately 33 clinical trials, some of the examples are: NCT04539262 (Phase 2) NCT04610541 (Phase 3) NCT04501952 (Phase 3) NCT04292730 (Phase 3) NCT04560231 (Phase 1) NCT04672564 (Phase 3) NCT04480333 (Phase 1) |
Phase3/NCT04292899/completed/March 6, 2020–April 9, 2020 | |
|
Ciclesonide
|
Glucocorticoid receptor inhibitor | Obstructive airway diseases. Asthma, Allergic Rhinitis, Perennial | Inhibiting the replication of SARS‐CoV‐2 genomic RNA via targeting viral endonuclease NSP15 |
NCT04330586 (Phase 2) NCT04435795 (Phase 3) NCT04377711 (Phase 3) NCT04381364 (Phase 2) NCT04356495 (Phase 3) |
Phase 3/NCT04377711/completed/June 8, 2020–January 5, 2021 |
(Kimura et al., 2020) |
|
Ribavirin
|
Nucleoside inhibitor | HIV infections, hepatitis C | RNA‐dependent RNA polymerase inhibitor |
Approximately 8 clinical trials, some of the examples are: NCT04828564(Phase 3) NCT04563208 (Phase 2) NCT04494399 (Phase 2) NCT04460443 (Phase 3) NCT04356677 (Phase 1) NCT04392427 (Phase 3) |
Phase 3/NCT04392427/not yet recruiting/October 2020–May 2022 | |
|
Dipyridamole
|
Phosphodiesterase inhibitor | Cirrhosis, hypertension, status; splenectomy, venous thrombosis, brain ischemia, transient ischemic attack, arteriosclerosis, | Suppressing HCoV‐19 replication |
NCT04391179 (Phase 2) NCT04424901 (Phase 2) NCT04410328 (Phase 3) |
Phase 3/NCT04410328/recruiting/October 21, 2020–March 15, 2021 |
(Liu, Li, Liu, Chen, & Luo, 2020; Liu, Zheng, Huang, Shan, & Huang, 2020; Ma & Wang, 2021) |
|
Ivermectin
|
Anti‐parasite agent, impα/β1‐mediated nuclear import inhibitor | Healthy, rosacea, lymphatic filariasis, loiasis |
Blocking α/β1‐mediated nuclear import |
Approximately 56 clinical trials, some of the examples are: NCT04529525 (Phase 3) NCT04381884 (Phase 2) NCT04523831 (Phase 3) NCT04391127 (Phase 3) NCT04602507 (Phase 2) NCT04422561 (Phase 3) NCT04530474 (Phase 3) |
Phase 3/NCT04523831/completed/June 1, 2020–August 22, 2020 | |
|
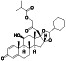
Rosuvastatin
|
HMG‐CoA reductase enzyme inhibitor, cholesterol level inhibitor, block hERG current |
Dyslipidemias, non‐ST‐elevation acute coronary syndromes, cardiovascular disease, myocardial infarction, hypercholesterolemia | Targeting COVID‐19 virus Mpro | NCT04472611 (Phase 3) | Phase 3/NCT04362137/not yet recruiting/August 1, 2020–August 1, 2021 |
(Farag et al., 2020) |
|
Isoquercetin (Quercetin 3‐glucoside, IQC‐950AN)
|
Regulates the expression of nitric oxide synthase 2 (NO2) via modulating the nuclear factor‐κB (NF‐κB) transcription regulation system | Cardiovascular disease, chronic kidney disease, Covid19 | Mpro inhibitor |
NCT04536090 (Phase 2) NCT04733651 (Phase 2) NCT04622865 (Phase 2) |
Phase 2/NCT04622865/recruiting/November 10, 2020–June 2021 | (Saeedi‐Boroujeni & Mahmoudian‐Sani, 2021) |
|
Ritonavir
|
HIV type 1aspartate protease inhibitor | HIV infection | Inhibit the coronaviral 3CLpro protease |
Approximately 24 clinical trials, some of the examples are: NCT04403100 (Phase 3) NCT04321174 (Phase 3) NCT04291729 (Phase 4) NCT04345276 (Phase 4) NCT04261270 (Phase 3) NCT04386876 (Phase 1) NCT04466241 (Phase 2) |
Phase 4/NCT04291729/completed/February 17, 2020–March 19, 2020 |
(Cao et al., 2020; Chu et al., 2004; Li & De Clercq, 2020; Ma et al., 2021; Sheahan, Sims, Leist, et al., 2020; Zhang, Lin, et al., 2020) |
|
Etoposide (VP‐16; VP‐16‐213)
|
Topoisomerase II inhibitor | Adult acute lymphocytic leukemia, NK + T Cell lymphoma, mantle cell lymphoma, small cell lung cancer | SARS‐3CL protease inhibitor | NCT04356690 (Phase 2) | Phase 2/NCT04356690/Active, not recruiting/April 22, 2020–December 2021 | (Rashid et al., 2022) |
|
PF‐07321332
|
SARS‐CoV 3C‐like protease (3CLPRO) inhibitor | COVID‐19 | 3CLpro inhibitor |
NCT04960202 (Phase 3) NCT04962230 (Phase 1) NCT05005312 (Phase 1) NCT04909853 (Phase 1) NCT04962022 (Phase 1) NCT05032950 (Phase 1) NCT04756531 (Phase 1) NCT05011513 (Phase 3) |
Phase 3/NCT04960202/recruiting/July 13, 2021–October 16, 2021 | (Vandyck & Deval, 2021) |
|
PF‐07304814
|
Potent 3CLpro protease (Mpro) inhibitor | Viral disease | 3CLpro proteaseinhibitor |
NCT04535167 (Phase 1) NCT04627532 (Phase 1) |
Phase 1/NCT04627532/completed/November 13, 2020–December 17, 2020 | (Boras, 2021; Vandyck & Deval, 2021) |
|
Lopinavir
|
HIV type 1 aspartate protease inhibitor | HIV infection |
Inhibit the viral proteases: 3CLpro or Plpro, increasing t1/2 via inhibiting cytochrome P450 when combined with lopinavir |
Approximately 18 clinical trials, some of the examples are: NCT04403100 (Phase 3) NCT04307693 (Phase 2) NCT04321174 (Phase 3) NCT04455958 (Phase 2) NCT04255017 (Phase 4) NCT04330690 (Phase 2) NCT04386876 (Phase 1) |
Phase 4/NCT04255017/recruiting/February 1, 2020–June 1, 2020 |
(Cao et al., 2020; Chu et al., 2004; Li & De Clercq, 2020; Ma et al., 2021; Sheahan, Sims, Leist, et al., 2020; Zhang, Lin, et al., 2020) |
|
Oseltamivir
|
Virus neuraminidase enzyme inhibitor | Influenza A and B viruses | Virus‐release blockers | NCT04303299 (Phase 3) NCT04558463 (Phase 3) NCT04516915 (Phase 3) NCT04255017 (Phase 4) NCT02735707 (Phase 4) NCT04338698 (Phase 3) | Phase 4/NCT04255017/recruiting/February 1, 2020–June 1, 2020 |
(Akram et al., 2020; Clinical trial ID: NCT04255017, n.d.; Whitley, 2007) |
|
Favipiravir
|
Viral RNA polymerase inhibitor | Influenza |
RNA dependent RNA polymerase inhibitor, reducing virus replication |
Approximately 38 clinical trials, some of the examples are: NCT04402203 (Phase 3) NCT04464408 (Phase 3) NCT04359615 (Phase 4) NCT04303299 (Phase 3) NCT04529499 (Phase 3) NCT04349241 (Phase 3) NCT04600999 (Phase 3) NCT04613271 (Phase 3) |
Phase 4/NCT04359615/not yet recruiting/April 20, 2020–May 3, 2020 | |
|
Ledipasvir
|
Inhibitor of the hepatitis C virus NS5A |
Hepatitis C, hepatocellular carcinoma | RdRP inhibitor | NCT04498936 (Phase 4) NCT04530422 (Phase 3) |
Phase 4/NCT04498936/ sofosbuvir/ledipasvir and nitazoxanide for treatment of COVID‐19 |
|
|
Sofosbuvir
|
HCV RNA replication inhibitor | Hepatitis C, liver cirrhosis | RdRP inhibitor |
Approximately 8 clinical trials, some of the examples are: NCT04530422 (Phase 3) NCT04498936 (Phase 4) NCT04497649 (Phase 3) NCT04532931 (Phase 2) NCT04535869 (Phase 3) NCT04561063 (Phase 2) NCT04460443 (Phase 3) |
Phase 4/NCT04498936/completed/July 15, 2020–October 30, 2020 | |
|
GS‐441524
|
Inhibits feline infectious peritonitis virus (FIPV), parent nucleoside of remdesivir | COVID‐19 |
Interferes with the SARS‐CoV‐2 RNA‐dependent RNA polymerase (RdRp) |
NCT04859244 (Phase 1) | Phase 1/NCT04859244/completed/April 26, 2021–May 1, 2021 | (Yan & Muller, 2020) |
|
AT‐527 (R07496998)
|
HCV viral replication inhibitor | HCV Infection | Inhibit the RdRp for RNA synthesis |
NCT04889040 (Phase 3) NCT04709835 (Phase 2) NCT04396106 (Phase 2) NCT04877769 (Phase 1) |
Phase 3/NCT04889040/recruiting/May 17, 2021–August 3, 2021 | (Good et al., 2021) |
|
Darunavir
|
HIV protease inhibitor | HIV infections, healthy | Protease inhibitor |
NCT04252274 (Phase 3) NCT04435587 (Phase 4) NCT04303299 (Phase 3) |
Phase 4/NCT04435587/recruiting/July 13, 2020–June 2021 |