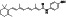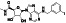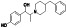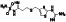TABLE 3.
Addressing the cytokine storm syndrome
| Drug name and structure | Original mechanism | FDA‐approved indication(s) | Possible mechanism for anti‐COVID‐19 | Clinical trials ID (stage) | The highest anti‐COVID‐19 phase/ID/status/start–(estimated)end dates | References |
|---|---|---|---|---|---|---|
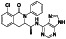
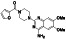
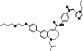
|
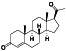
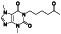
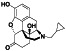
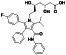
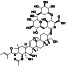
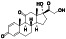
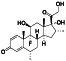
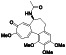
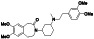
Progesteron
|
Steroid hormone regulating menstrual cycle | Suicidal ideation, infertility, hormone replacement, concussion | Inhibiting pro‐inflammatory cytokines IL‐1β and IL 12 | NCT04365127 (Phase 1) | Phase1/NCT04365127/completed/April 27, 2020–August 20, 2020 |
(Mauvais‐Jarvis et al., 2020) |
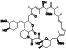
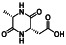
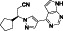
|
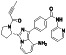
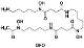
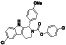
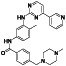
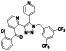
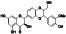
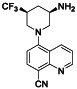
Acalabrutinib
|
BTK inhibitor | Not yet approved | BTK inhibitor, decreased inflammatory cell infiltration and proinflammatory cytokines | NCT04380688(Phase 2) NCT04346199(Phase 2) NCT04497948(Phase 1) NCT04564040(Phase 1) | Phase 2/NCT04346199/completed/June 12, 2020–November 17, 2020 |
(Roschewski et al., 2020) |
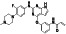
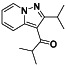
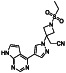
|
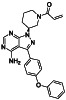
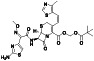
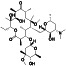
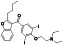
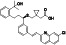
Ibrutinib
|
Irreversible BTK inhibitor |
Lymphocytic, waldenstrom macroglobulinemia, mantle‐cell, B‐cell, etc. |
BTK inhibitor, decreasing inflammatory cell infiltration and proinflammatory cytokines |
NCT04375397 (Phase 2) NCT04665115 (Phase 2) NCT04439006 (Phase 2) |
Phase 2/NCT04439006/recruiting/July 22, 2020–December 31, 2021 |
(Treon et al., 2020) |
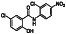
|
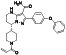
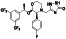
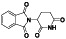
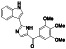
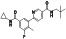
Zanubrutinib
|
Selective BTK inhibitor | Not approved yet | BTK inhibitor, decreased inflammatory cell infiltration and proinflammatory cytokines | NCT04382586 (Phase 2) | Phase 2/NCT04382586/active, not recruiting/July 6, 2020–February 2, 2021 |
(Chong et al., 2020) |
|
Duvelisib
|
Selectivite p100δ inhibitor | Not approved yet |
Inhibiting phosphatidylinositol 3‐kinase(PI3K) which plays acrucial role in eliciting immune response. |
NCT04372602 (Phase 2) NCT04487886 (Phase 2) |
Phase 2/NCT04372602/recruiting/October 12, 2020–November 30, 2021 |
(Clinical trial ID: NCT04487886, n.d.) |
|
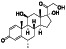
Sirolimus (rapamycin)
|
mTOR inhibitor | Kidney transplantation, blue rubber bleb nevus syndrome, venous malformation, kidney transplantation, uterine fibroids |
Alleviating cytokine strom and T‐cell senescence, preventing severe COVID‐19 progression |
Approximately 7 clinical trials, some of the examples are: NCT04461340 (Phase 2) NCT04371640 (Phase 1) NCT04341675 (Phase 2) NCT04409327 (Phase 2) |
Phase 2/NCT04341675/recruiting/April 24, 2020–July 2020 |
(Omarjee et al., 2020) |
| ArtemiC (artemisinin, curcumin, frankincense and vitamin C) | Anti‐inflammation | Not currently approved | Diminishing activity of TNF‐α and IL‐6 levels | NCT04382040 (Phase 2) | Phase 2/NCT04382040/completed/May 8, 2020–November 2020/MGC pharmaceuticals d.o.o) |
(Clinical trial ID: NCT04382040, n.d.) |
|
Abivertinib
|
Tyrosine kinase inhibitor (TKI), BTK Inhibitor, third‐generation EGFR tyrosine kinase inhibitor | Not yet approved | Inhibition of vital pro‐inflammatory cytokine production, such as IL‐6, IL‐1β and TNF‐α |
NCT04528667 (Phase 2) NCT04440007 (Phase 2) |
Phase 2/NCT04528667/recruiting/September 2020–May 2021 |
(Clinical trial ID: NCT04440007, n.d.; Sorrento Therapeutics Inc, News Release, 2020) |
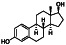
|
Estradiol
|
Enhancing expression of interleukin 6 via the estrogen receptor β (ERβ) pathway |
Menorrhagia, vaginal atrophy, healthy, suicidal ideation |
Anti‐inflammatory and immunomodulatory, blocking the proinflammatory cytokines production, such as IL‐6, IL‐1β, TNF‐α and chemokine CCL2 |
NCT04359329 (Phase 2) | Phase 2/NCT04359329/recruiting/April 20, 2020–November 15, 2020 |
(Mauvais‐Jarvis et al., 2020) |
|
Pentoxifylline
|
Competitive nonselective phosphodiesterase inhibitor | Type 2 diabetes, chronic renal failure, severe alcoholic hepatitis, lupus nephritis, irritable bowel syndrome, diabetic kidney disease et al. |
Anti‐inflammatory, blocking the proinflammatory cytokines production, such as IL‐6, IL‐1, TNF‐α, C‐reactive protein and other immunoregulators. |
NCT04433988 (Phase 2) NCT04570254 (not applicable) |
Phase 2/NCT04433988/not yet recruiting/December 13, 2020–December 30, 2021 |
(Assimakopoulos et al., 2020; González‐Pacheco et al., 2020) |
|
Deferoxamine
|
Iron chelator | Iron overload, diabetic foot ulcer, acute ischemic stroke, Beta‐Thalassemia, |
Inhibiting IL‐6 synthesis through decreasing NF‐kB. |
NCT04333550 (Phase 2) NCT04361032 (Phase 3) NCT04389801 (Phase 4) |
Phase 4/NCT04389801/Not yet recruiting/May 15, 2020–September 30, 2020 | (Clinical trial ID: NCT04389801, n.d.; Dalamaga et al., 2020) |
|
Cefditoren (Pivoxil, ME 1207)
|
New‐third generation cephalosporin antibiotic, broad spectrum of activity against Gram‐positive and gram‐negative bacteria | Rhinosinusitis, COVID‐19 pneumonia, urinary tract infections | Decrease IL‐6 level and other pro‐inflammatory cytokines | NCT04709172 (Phase 4) | Phase 4/NCT04709172/recruiting/January 14, 2021–April 5, 2021 |
(Clinical trial ID: NCT04709172, n.d.) |
|
Aprepitant (cinvanti)
|
Neurokinin 1 receptor antagonist | Postoperative nausea and vomiting, healthy volunteers, emesis, breast cancer, leukemia |
Anti‐inflammatory, blocking inflammatory cytokines release mediated by the binding of substance P to NK1 receptors |
NCT04470622 (Phase 2) |
Phase 2/NCT04470622/recruiting/July 20, 2020–June 2021/Heron Therapeutics |
(Clinical trial ID: NCT04470622, n.d.; Mehboob et al., 2020) |
|
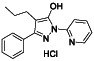
Prazosin
|
Selective alpha 1‐adrenergic blocking agent. | PTSD, panic disorder, hypertension and anxiety and anxiety | α‐1 adrenergic receptor antagonist, prevent cytokine storm | NCT04365257 (Phase 2) | Phase 2/NCT04365257/recruiting/May 13, 2020–June 30, 2021 |
(Konig et al., 2020) |
|
Ampion (DA‐DKP, aspartyl‐alanyl diketopiperazine)
|
Modulating inflammatory immune response via molecular pathways related to T lymphocyte energy deficiency |
Inflammation associated with osteoarthritis | Anti‐inflammatory, blocking the proinflammatory cytokines production in T‐cells |
NCT04456452 (Phase 1) NCT04839965 (Phase 2) NCT04606784(Phase 1) |
Phase2/NCT04839965/recruiting/April 2021–October 2021 |
(Clinical trial ID: NCT04414618, n.d.) |
|
Ibudilast
|
Nonselective phosphodiesterase inhibitor | Asthma, multiple sclerosis | Anti‐inflammatory | NCT04429555 (Phase 2) | Phase 2/NCT04429555/recruiting/November 2020–June 30, 2021 |
(Clinical trial ID: NCT04429555, n.d.) |
|
LAU‐7b (fenretinide [4‐HPR])
|
Retinoic acid receptors (RAR) inhibitor | Not approved yet | Anti‐inflammatory and antiviral activities | NCT04417257 (Phase 2) | Phase 2/NCT04417257/recruiting/June 29, 2020–May 15, 2021 |
(Orienti et al., 2020) |
|
Naltrexone
|
Blockade of opioid receptors, κ‐opioid receptor antagonist | Alcoholism, opioid use, obesity | Interrupt the inflammation |
NCT04365985 (Phase 2) NCT04756128 (Phase 2) NCT04604678 (Phase 2) NCT04604704 (Phase 2) NCT04708327 (Phase 2) |
Phase 2/NCT04365985/recruiting/April 29, 2020–May 2021 |
(Choubey et al., 2020) |
|
Piclidenoson
|
Adenosine A3 receptor agonist | Not approved yet | Anti‐inflammatory | NCT04333472 (Phase 2) | Phase 2/NCT04333472/recruiting/January 6, 2021–March 6, 2022 |
(David & Shweta, 2020) |
|
Clarithromycin
|
Macrolide antibiotic and a CYP3A4 inhibitor | Antibiotics‐bacterial infection, nontuberculous mycobacteria, mycobacterium avium complex, crohn's disease, ulcer, peptic ulcer, periodontitis | Anti‐inflammatory nature and enhance the immunity | NCT04398004 (Phase 2) NCT04622891 (not applicable) | Phase 2/NCT04398004/completed/May 6, 2020–November 30, 2020 | |
|
Thalidomide
|
Cereblon (CRBN) inhibitor, sedative, part of the cullin‐4 E3 ubiquitin ligase complex CUL4‐RBX1‐DDB1 |
Mantle cell lymphoma, NSCLC, congestive heart failure, multiple myeloma, gastric cancer | Anti‐inflammatory and anti‐fibrosis | NCT04273581 (Phase 2) NCT04273529 (Phase 2) | Phase 2/NCT04273581/ot yet recruiting/February 18, 2020–April 30, 2020 |
(Khalil et al., 2020) |
|
Cenicriviroc
|
Dual CCR2/CCR5 antagonist | Not yet approved | Anti‐inflammatory and immunomodulatory effects | NCT04500418 (Phase 2) | Phase 2/NCT04500418/recruiting/August 25, 2020–September 30, 2021 |
(Okamoto et al., 2020) |
|
Ruxolitinib
|
Selective JAK1/2 inhibitor | Thalassemia major, splenomegaly | Inhibit the downstream IFNg pathway targeting JAK kinase receptor |
Approximately 19 clinical trials, some of the examples are: NCT04414098 (Phase 2) NCT04477993 (Phase 3) NCT04581954 (Phase 2) NCT04362137 (Phase 3) NCT04348071 (Phase 3) NCT04377620 (Phase 3) NCT04338958 (Phase 2) |
Phase 3/NCT04362137/completed/May 2, 2020–October 17, 2020 | |
|
Baricitinib
|
JAK1 and JAK2 inhibitor | Rheumatoid arthritis |
JAK–STAT signal blocking |
Approximately 11 clinical trials, some of the examples are: NCT04358614 (Phase 3) NCT04320277 (Phase 3) NCT04340232 (Phase 3) NCT04373044 (Phase 2) NCT04421027 (Phase 3) NCT04346147 (Phase 2) NCT04832880 (Phase 3) |
Phase 3/NCT04421027/recruiting/June 12, 2020–May 19, 2021 | |
|
Niclosamide
|
Anti‐parasitic drug, STAT3 inhibitor, DNA replication inhibitor | Not yet approved |
Blocking signaling pathways including mTORC1, IL‐6‐JAK1‐STAT3 signaling |
Approximately 13 clinical trials, some of the examples are: NCT04558021 (Phase 3) NCT04399356 (Phase 2) NCT04542434 (Phase 2) NCT04372082 (Phase 3) NCT04603924 (Phase 3) NCT04436458 (Phase 2) NCT04524052 (Phase 1) |
Phase 3/NCT04558021/recruiting/October 8, 2020–January 30, 2021 |
(Pindiprolu & Pindiprolu, 2020; Xu, Shi, Wang, et al., 2020) |
|
Levamisole
|
Anthelmintic and immunomodulator | Broad‐spectrum insect repellent |
Down regulating the level of IL‐6 and TNF‐α |
NCT04360122 (Phase 3) NCT04383717 (Phase 3) NCT04331470 (Phase 3) |
Phase 3/NCT04331470/recruiting/April 4, 2020–April 20, 2020 |
(Uyaroğlu et al., 2020) |
|
Opaganib
|
Selective, competitive sphingosine kinase 2 (SK2) inhibitor | Not yet approved |
Down regulating the level of IL‐6 and TNF‐α, inhibiting viral replication |
NCT04414618 (Phase 2) NCT04467840 (Phase 3) NCT04502069 (Phase 2) |
Phase 3/NCT04467840/recruiting/August 21, 2020–June 2021 |
(Kurd et al., 2020) |
|
Anakinra
|
Interleukin‐1 receptor (IL‐1) antagonist | Rheumatoid arthritis, knee injuries | Interleukin‐1 (IL‐1) blockage |
Approximately 17 clinical trials, some of the examples are: NCT04826588 (Phase 3) NCT04364009 (Phase 3) NCT04443881 (Phase 3) NCT04603742 (Phase 2) NCT04357366 (Phase 2) NCT04339712 (Phase 2) NCT04412291 (Phase 2) |
Phase 3/NCT04443881/recruiting/May 8, 2020–December 2020 |
(Gallelli et al., 2020) |
|
PF‐06650833
|
Interleukin‐1 receptor associated kinase 4 (IRAK4) inhibitor | Rheumatoid arthritis, lupus, lymphomas | Inhibition of interleukin‐1 receptor associated kinase 4 (IRAK4) in ameliorating the proinflammatory state and improving outcomes in severe COVID‐19. | NCT04933799 (Phase 2) | Phase 2/NCT04933799/recruiting/June 22, 2021–March 6, 2022 | (Clinical trial ID: NCT04933799, n.d.) |
|
Atorvastatin
|
HMG‐CoA reductase inhibitor | Stable angina, acute coronary syndrome, hepatocellular carcinoma, cardiovascular disease, ischemia et al. | lower levels of inflammatory cytokines |
NCT04466241 (Phase 2) NCT04380402 (Phase 2) NCT04486508 (Phase 3) |
Phase 3/NCT04486508/active, not recruiting/July 30, 2020–April 4, 2021 |
(Ghati et al., 2020; Li, 2020; Tan et al. 2020) |
|
PTC299
|
Dihydroorotate dehydrogenase (DHODH) inhibitor | Not yet approved |
Blocking the proinflammatory cytokines production such as IL‐6, IL‐17, IL‐17F |
NCT04439071 (Phase 3) | Phase 3/NCT04439071/recruiting/July 9, 2020–January 30, 2021 |
(Luban et al., 2021) |
|
Amiodarone
|
ATP‐sensitive potassium channel inhibitor | Atrial fibrillation, atrial fibrillation, new oonset atrial fibrillation, paroxysmal atrial fibrillation, ventricular Tachycardia et al. |
NCT04351763 (Phase 3) |
Phase 3/NCT04351763/recruiting/April 27, 2020–March 2, 2021 |
(Sanchis‐Gomar et al., 2020) |
|
|
VERU‐111
|
Orally bioavailable α and β tubulin inhibitor | Metastatic castration resistant prostate cancer | Anti‐inflammatory action against the key cytokines involved in the cytokine storm | NCT04388826 (Phase 2) NCT04842747 (Phase 3) | Phase 3/NCT04842747/not yet recruiting/April 30, 2021–January 5, 2022 |
(Clinical trial ID: NCT04388826, n.d.) |
|
Escin
|
Vasoprotective anti‐inflammatory, anti‐nociceptive and anti‐edematous agent. | Chronic venous insufficiency | Vasoprotective anti‐inflammatory | NCT04322344 (Phase 2) | Phase 3/NCT04322344/recruiting/March 23, 2020–June 30, 2020 |
(Clinical trial ID: NCT04322344, n.d.; Gallelli et al., 2020) |
|
Imatinib
|
Tyrosine kinases inhibitor |
Breast cancer, gastrointestinal stromal tumors, chronic myeloid leukemia |
Tyrosine kinase inhibitors in the regulation of inflammation |
NCT04794088 (Phase 2) NCT04422678 (Phase 3) NCT04394416 (Phase 3) NCT04357613 (Phase 2) NCT04346147 (Phase 2) |
Phase 3/NCT04394416/recruiting/June 2, 2020–June 1, 2022 |
(Sisk et al., 2018) |
|
Montelukast
|
Cysteinyl leukotriene receptor 1 (Cysltr1) antagonist | Asthma and liver injury, reduce cardiac damage |
Anti‐inflammatory, reducing production of cytokine |
NCT04714515 (Phase 3) NCT04389411 (Phase 3) NCT04695704 (Phase 3) NCT04718285 (Phase 2) |
Phase 3/NCT04714515/completed/February 20, 2020–March 30, 2020 | |
|
Losmapimod
|
p38 MAPK inhibitor | Not yet approved | Reduce the acute exaggerated pro‐inflammatory responses | NCT04511819 (Phase 3) | Phase 3/NCT04511819/active, not recruiting/August 28, 2020–June 2021 |
(Clinical trial ID: NCT04511819, n.d.) |
|
Tradipitant
|
Neurokinin‐1 (NK‐1) antagonist | Eczema, pruritus, gastroparesis, chronic pruritus, and atopic dermatitis | NK‐1 antagonist involved in neuroinflammatory processes | NCT04326426 (Phase 3) | Phase 3/NCT04326426/enrolling by invitation/April 13, 2020–August 1, 2020 |
(Clinical trial ID: NCT04326426, n.d.) |
|
Ifenprodil (NP‐120)
|
Noncompetitive N‐methyl‐d‐aspartate (NMDA) receptor antagonist | Posttraumatic stress disorders |
NDMA receptor‐type subunit 2B antagonist and the subunit receptor mainly expressed on T cells and neutrophils |
NCT04382924 (Phase 3) | Phase 3/NCT04382924/active, not recruiting/August 5, 2020–January 2022 |
(Clinical trial ID: NCT04382924, n.d.) |
|
Prednisone
|
Synthetic corticosteroid agent, upregulating natriuretic peptide receptor type A | Comparative study, immunosuppressive agents, inflammatory bowel disease, multiple sclerosis |
Agonist of glucocorticosteroid, blocking inflammatory and immune responses. |
NCT04534478 (Phase 4) NCT04344288 (Phase 2) |
Phase 4/NCT04534478//not yet recruiting/September 7, 2020–May 2, 2021 |
(Herman et al., 2020) |
|
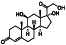
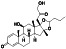
Hydrocortisone
|
Human endogenous metabolite, steroid hormone or glucocorticoid secreted by the adrenal cortex | Coronary artery disease, healthy, septic shock, asthma |
Agonist of glucocorticosteroid, blocking inflammatory and immune responses. |
NCT04348305 (Phase 3) NCT02735707 (Phase 4) NCT02517489 (Phase 3) NCT04599959 (not applicable) NCT04563676 (not applicable) | Phase 4/NCT02735707/recruiting/April 11, 2016–December 2021 | |
|
Dexamethasone
|
Glucocorticoid receptor agonist | Cervical radiculopathy, cancer, inflammatory response, hip fracture, pain |
Agonist of glucocorticosteroid, reducing CD11b, CD18, and CD62L |
Approximately 31 clinical trials, some of the examples are: NCT04834375 (Phase 4) NCT04603729 (Phase 3) NCT04499313 (Phase 3) NCT04325061 (Phase 4) NCT04325061 (Phase 4) NCT04347980 (Phase 3) NCT04563494 (Phase 4) NCT03170882 (Phase 2) |
Phase 4/NCT04834375/recruiting/March 19, 2021–March 19, 2022 |
(Horby et al., 2021) |
|
Budesonide
|
Orally active glucocorticoid receptor agonist | Asthma | Glucocorticoid receptor agonist, Suppress the immune reaction locally in the respiratory system. | NCT04331470 (Phase 3) NCT04374474 (Phase 4) NCT04361474 (Phase 3) | Phase 4/NCT04374474/active, not recruiting/May 18, 2020–November 24, 2020 |
(De León‐Rendón et al., 2020) |
|
Famotidine
|
Competitive histamine H2‐receptor antagonist | Peptic ulcer, HIV infections, aspirin, dyspepsia, stomach ulcer, heartburn | Anti‐inflammatory action | NCT04836806 (Phase 4) NCT04504240 (Phase 3) NCT04545008 (Phase 1) NCT04565392 (Phase 4) NCT04724720 (Phase 2) NCT04370262 (Phase 3) NCT04389567 (not applicable) | Phase 4/NCT04565392/not yet recruiting/May 1, 2021–November 1, 2021 |
(Ennis & Tiligada, 2021) |
|
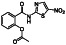
Nitazoxanide
|
Antiprotozoal agent |
Irritable bowel syndrome, hepatitis C, diabetes mellitus type 2 |
Control excessive inflammatory immune responses |
Approximately 24 clinical trials, some of the examples are: NCT04406246 (Phase 4) NCT04552483 (Phase 2) NCT04498936 (Phase 4) NCT04463264 (Phase 3) NCT04486313 (Phase 3) NCT04532931 (Phase 2) NCT04563208 (Phase 2) |
Phase 4/NCT04498936/completed/July 15, 2020–October 30, 2020 |
(Kelleni, 2020; Mahmoud et al., 2020; Pepperrell et al., 2020) |
|
Colchicine
|
Tubulin inhibitor and microtubule disrupting agent | Myocardial infarction, intercritical gout | Anti‐inflammatory effects, an inhibitor of NLRP3 inflammasomes and mitigating interleukin activation. |
Approximately 27 clinical trials, some of the examples are: NCT04818489 (Phase 4) NCT04392141 (Phase 2) NCT04416334 (Phase 3) NCT04472611 (Phase 3) NCT04322565 (Phase 2) NCT04724629 (Phase 3) NCT04492358 (Phase 3) |
Phase 4/NCT04818489/recruiting/March 25, 2021–May 25, 2021 | |
|
Silymarin
|
p38 MAPK pathway inhibitor | Non‐alcoholic fatty liver disease, metastatic colorectal cancer, hepatitis | Anti‐inflammatory and anti‐oxidant effects |
NCT04394208 (Phase 3) NCT04816682 (Phase 4) |
Phase 4/NCT04816682/recruiting/March 17, 2021–June 30, 2021 |
(Clinical trial ID: NCT04394208, n.d.) |
|
Methylprednisolone
|
Synthetic corticosteroid, anti‐inflammatory and immunomodulating properties | COPD, purpura, schoenlein‐henoch, postoperative pain, drug interaction potentiation, et al. |
Anti‐inflammatory, decreasing level of IL‐6, ACE2 activation |
Approximately 15 clinical trials, some of the examples are: NCT04499313 (Phase 3) NCT04485429 (Phase 3) NCT04603729 (Phase 3) NCT04826588 (Phase 3) NCT03708718 (Phase 2) NCT04780581 (Phase 4) NCT04765371 (Phase 3) |
Phase 4/NCT04780581/recruiting/February 1, 2021–December 1, 2021 |
(Edalatifard et al., 2020; Liu, Zheng, Huang, Shan, & Huang, 2020) |
|
Enpatoran (M5049)
|
Dual TLR7/8 inhibitor | Systemic lupus erythematosus, Coronavirus Disease 2019 | Blocks the activation of toll‐like receptor (TLR)7 and TLR8, reduction in the inflammatory response | NCT04448756 (Phase 2) | Phase 2/NCT04448756/Active, not recruiting/June 26, 2020–August 22, 2021 | (Clinical trial ID: NCT04448756, n.d.) |
|
EC‐18
|
Anti‐inflammation | Stomatitis, chemotherapy‐Induced neutropenia, febrile neutropenia | Anti‐inflammatory, prevention of COVID‐19 infection to severe pneumonea or ARDS |
NCT04500132 (Phase 2) NCT04569227 (Phase 2) |
Phase 2/NCT04569227/recruiting/September 29, 2020–September 2021 | (Clinical trial ID: NCT04569227, n.d.) |
|
APX‐115 (Ewha‐18,278)
|
pan NADPH oxidase (Nox) inhibitor | Healthy, diabetic nephropathies |
NADPH‐dependent generation of superoxide and secondary reactive oxygen species (ROS). ROS are often generated during virus infection, thus promoting apoptosis, lung injury, and inflammation/allergy. |
NCT04880109 (Phase 2) | Phase 2/NCT04880109/not yet recruiting/May 10, 2021–February 2022 |
(Clinical trial ID: NCT04880109, n.d.) |