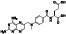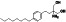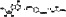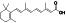TABLE 4.
Modulating immune system
| Drug name and structure | Original mechanism | FDA‐approved indication(s) | Possible mechanism for anti‐COVID‐19 | Clinical trials ID (stage) | The highest anti‐COVID‐19 phase/ID/status/start–(estimated)end dates | References |
|---|---|---|---|---|---|---|
|
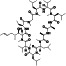
Methotrexate
|
Enzyme dihydrofolate reductase inhibitor, an immunosuppressant and antineoplastic agent | Rheumatoid arthritis, ophthalmopathy, thyroid‐associated, psoriatic arthritis |
Immunomodulatory agent, preventing excessive immunereaction |
NCT04352465 (Phase 2) NCT04610567 (Phase 2) |
Phase 2/NCT04610567/recruiting/October 27, 2020–March 15, 2021 |
(Abuo‐Rahma et al., 2020) |
|
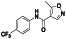
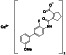
Leflunomide
|
Dihydroorotate dehydrogenase inhibitor | Rheumatoid arthritis, psoriatic arthritis, immunoglobulin G4 related sclerosing disease, juvenile idiopathic arthritis |
Immunomodulatory agent, preventing excessive immunereaction |
NCT04532372 (Phase 2) NCT04361214 (Phase 1) |
Phase 2/NCT04532372/recruiting/January 7, 2021–September 18, 2022 |
(Abuo‐Rahma et al., 2020) |
|
Fingolimod
|
Sphingosine 1‐phosphate (S1P) antagonist, pak1 activator, a immunosuppressant | Multiple sclerosis, relapsing–remitting |
Immune modulator, S1P1 receptors antagonist |
NCT04280588 (Phase 2) |
Phase 2/NCT04280588/withdrawn (no participants enrolled)/February 22, 2020–July 1, 2020 |
|
|
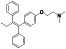
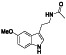
Tamoxifen
|
Selective estrogen receptor modulator (SERM), Hsp90 activator | Breast cancer, infertility, menorrhagia, endometrium | Modulation of NK cells activity and reduce viral replication, through reducing PGE2 production |
NCT04389580 (Phase 2)NCT04568096 (Phase 2) |
Phase 2/NCT04568096/not yet recruiting/November 2020–December 2020 | |
|
Isoprinosine
|
Immunostimulant | Immunostimulant | Immunostimulant |
NCT04360122 (Phase 3) NCT04383717 (Phase 3) |
Phase 3/NCT04360122/not yet recruiting/May 20, 2020–November 1, 2020 |
(Clinical trial ID: NCT04360122, n.d.; Kumar et al., 2020) |
|
IMU‐838 (vidofludimus calcium)
|
Immunomodulatory agent, DHODH inhibitor, inhibiting IL‐17 secreting |
Not yet approved | Selective immunomodulatory effect against highly activated immune cells | NCT04516915 (Phase 2) NCT04379271 (Phase 3) | Phase 3/NCT04379271/recruiting/June 11, 2020–September 2020 |
(Clinical trial ID: NCT04379271, n.d.; Immunic Therapeutics website) |
|
All‐trans retinoic acid
|
Neutrophil elastase inhibitor, RAR nuclear receptor agonist | Acute promyelocytic leukemia | Enhance neutralizing antibodies |
NCT04396067 (Phase 2) NCT04568096 (Phase 2) NCT04730895 (Phase 2) NCT04578236 (Phase 2) NCT04353180 (Phase 3) |
Phase 3/NCT04353180/not yet recruiting/April 2021–June 2021 |
(Clinical trial ID: NCT04396067, n.d.) |
|
Melatonin
|
Selective ATF‐6 inhibitor | Parkinson's disease, infertility, immediate dental implant, immediate dental implant, postoperative pain, anxiety, acute ischemic stroke | Modulate the immune response and neuroinflammation |
Approximately 8 clinical trials, some of the examples are: NCT04474483 (Phase 2) NCT04470297 (Phase 2) NCT04568863 (Phase 2) NCT04353128 (Phase 3) NCT04409522 (not applicable) NCT04531748 (Phase 2) NCT04530539 (not applicable) |
Phase 3/NCT04353128/recruiting/April 20, 2020–October 2020 |
(Bahrampour Juybari et al., 2020) |
|
Cyclosporinea(Synonyms: cyclosporine; Ciclosporin)
|
Immunosuppressant, inhibiting CD11a/CD18 adhesion | End‐stage renal disease, renal function and chronic allograft vasculopathy, kidney transplantation, etc |
Immunomodulatory agent acting on T cells |
NCT04540926 (Phase 2) NCT04412785 (Phase 1) NCT04492891 (Phase 2) NCT04392531 (Phase 4) NCT04451239 (not applicable) | Phase 4/NCT04392531/recruiting/April 16, 2020–March 2021 | |
|
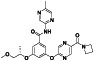
AZD1656
|
Glucokinase activator | Type II diabetes | Activate the migration of T regulatory cells to sites of inflammation | NCT04516759 (Phase 2) | Phase 2/NCT04516759/completed/August 18, 2020–April 25, 2021 | (Clinical trial ID: NCT04516759, n.d.) |