TABLE 5.
Anticoagulant therapy
| Drug name and structure | Original mechanism | FDA‐approved indication(s) | Possible mechanism for anti‐COVID‐19 | Clinical trials ID (stage) | The highest anti‐COVID‐19 phase/ID/status/start–(estimated)end dates | References |
|---|---|---|---|---|---|---|
|
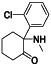
Ketamine
|
Antagonist of N‐methyl D‐aspartate receptor | Anesthesia, pain relief, sedation, and memory loss | Anesthesia, pain relief | NCT04365985 (Phase 2) | Phase 2/NCT04365985/recruiting/April 29, 2020–May 2021 |
(Suri & Sindwani, 2020) |
|
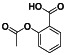
Aspirin
|
Non‐selective and irreversible COX‐1 and COX‐2 inhibitor | Common cold, healthy, acute coronary syndrome, et al. |
Interfering with normal platelet aggregation, COX‐1 inhibitor |
NCT04365309 (Phase 3) NCT04363840 (Phase 2) NCT04343001 (Phase 3) NCT04410328 (Phase 3) NCT04324463 (Phase 3) NCT04808895 (Phase 3) | Phase 3/NCT04410328/recruiting/October 21, 2020–March 15, 2021 |
(Clinical trial ID: NCT04365309, n.d.; Bianconi et al., 2020) |
|
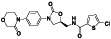
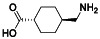
Rivaroxaban
|
Selective and direct Factor Xa (FXa) inhibitor | Atherosclerosis, mitral valve stenosis|atrial fibrillation | Adequate antithrombotic therapy | NCT04504032 (Phase 2) NCT04508023 (Phase 3) NCT0441604 (Phase 3) NCT04324463 (Phase 3) | Phase 3/NCT04324463/recruiting/April 21, 2020–December 31, 2020 |
(Di Tano et al., 2020) |
|
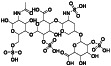
Enoxaparin
|
Anticoagulant medication |
Pulmonary embolism (PE), deep vein thrombosis (DVT) |
Adequate antithrombotic therapy |
Approximately 8 clinical trials, some of the examples are: NCT04427098 (Phase 2) NCT04366960 (Phase 3) NCT04400799 (Phase 3) NCT04540926 (Phase 2) NCT04492254 (Phase 3) NCT04408235 (Phase 3) NCT04640181 (Phase 2) |
Phase 3/NCT04400799/recruiting/June 15, 2020–March 14, 2021 |
(Drago et al., 2020) |
|
Tranexamic acid
|
Antifibrinolytic agent, blocking lysine‐binding sites of plasmin and elastase‐derived plasminogen fragments |
Prevent blood loss in surgical procedure | Antifibrinolytic agent, that competitively inhibits activation of plasminogen to plasmin, an enzyme that degrades fibrin clots. | NCT04338126 (Phase 2) NCT04550338 (Phase 3) NCT04338074 (Phase 2) | Phase 3/NCT04550338/not yet recruiting/December 1, 2020–December 31, 2022 |
(Ogawa & Asakura, 2020) |
|
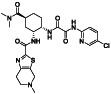
Edoxaban
|
FXa inhibitor | Atrial fibrillation (AF), venous thrombosis|neoplasms|anticoagulant, ischemic stroke, blood coagulation disorder, coronary artery disease | Anticoagulant activity of direct FXa inhibitors |
NCT04516941 (Phase 3) NCT04542408 (Phase 3) |
Phase 3/NCT04542408/recruiting/November 12, 2020–May 31, 2021 |
(Al‐Horani, 2020) |
|
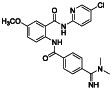
Apixaban
|
FXa inhibitor |
Deep vein thrombosis|pulmonary embolism, diabetes, venous thromboembolism, etc. |
Anticoagulant properties | NCT04512079 (Phase 4) | Phase 4/NCT04512079/recruiting/September 8, 2020–March 2021 |
(Al‐Horani, 2020) |