TABLE 6.
Antioxidant supplement
| Drug name and structure | Original mechanism | FDA‐approved indication(s) | Possible mechanism for anti‐COVID‐19 | Clinical trials ID (stage) | The highest anti‐COVID‐19 phase/ID/status/start–(estimated)end dates | References |
|---|---|---|---|---|---|---|
|
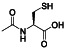
N‐acetylcysteine
|
ROS inhibitor, mucolytic agent reducing thickness of mucus, | Gastric mucosal lesion, polycystic ovary syndrome, etc. |
Antioxidant precursor to glutathione (γ‐glutamylcysteinylglycine), mucolytic agent |
NCT04374461 (Phase 2) NCT04792021 (Phase 3) NCT04545008 (Phase 1) NCT04458298 (Phase 2) NCT04419025 (Phase 2) NCT04703036 (Phase 1) NCT04755972 (not applicable) | Phase 3/NCT04792021/recruiting/March 9, 2021–June 2021 | |
|
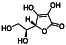
Vitamin C (ascorbic acid)
|
Effective reducing agent and donor antioxidant | Scurvy, upper respiratory tract infections, reduces the risk of lung cancer. |
Upregulating phagocytes and lymphocytes activity, modulating immune system |
Approximately 24 clinical trials, some of the examples are: NCT04401150 (Phase 3) NCT04468139 (Phase 4) NCT04363216 (Phase 2) NCT04264533 (Phase 2) NCT02735707 (Phase 4) NCT04780061 (Phase 3) NCT04828538 (not applicable) |
Phase 4/NCT02735707/recruiting/11, 2016–December 2021 |
(Baladia et al., 2020) |
|
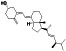
Vitamin D3 (Cholecalciferol)
|
Inducing cell differentiation and preventing the proliferation of cancer cells | Epilepsy, healthy, vitamin D deficiency, osteoporosis | Protect respiratory infections |
Approximately 14 clinical trials, some of the examples are: NCT04344041 (Phase 3) NCT04536298 (Phase 3) NCT04411446 (Phase 4) NCT04482673 (Phase 4) NCT04407286 (Phase 1) NCT04386850 (Phase 3) NCT04395768 (Phase 2) |
Phase 4/NCT04482673/recruiting/July 31, 2020–December 31, 2021 |
(Weir et al., 2020) |
|
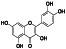
Quercetin
|
Stimulator of recombinant SIRT1, PI3K inhibitor | Menopause related conditions, mountain sickness, flushing | Prophylactic, antioxidant |
NCT04853199 (early Phase 1) NCT04578158 (Phase 2) NCT04468139 (Phase 4) NCT04536090 (Phase 2) NCT04377789 (not applicable) |
Phase 4/NCT04468139/recruiting/June 20, 2020–July 20, 2020 |
(Diniz et al., 2020) |