Abstract
Purpose:
Endocrine resistance remains a major clinical challenge in estrogen-receptor (ER) positive breast cancer. Despite the encouraging results from clinical trials for the drugs targeting known survival signaling, relapse is still inevitable. There is an unmet need to discover new drug targets in the unknown escape pathways. Here we report Nemo-Like Kinase (NLK) as a new actionable kinase target that endows previously uncharacterized survival signaling in endocrine resistant breast cancer.
Experimental Design:
The effects of NLK inhibition on the viability of endocrine resistant breast cancer cell lines were examined by MTS assay. The effect of VX-702 on NLK activity was verified by kinase assay. The modulation of ER and its coactivator SRC-3 by NLK were examined by immunoprecipitation, kinase assay, luciferase assay, and RNAseq. The therapeutic effects of VX-702 and Everolimus were tested on cell line- and patient-derived xenograft tumor models.
Results:
NLK overexpression endow reduced endocrine responsiveness and is associated with worse outcome of tamoxifen-treated patients. Mechanistically, NLK may function at last in part via enhancing the phosphorylation of ERα and its key coactivator SRC-3 to modulate ERα transcriptional activity. Through interrogation of a kinase-profiling database, we uncovered and verified a highly selective dual p38/NLK inhibitor, VX-702. Co-administration of VX-702 with the mTOR inhibitor Everolimus demonstrated a significant therapeutic effect in cell line- and patient-derived xenograft tumor models of acquired or de novo endocrine resistance.
Conclusions:
Together, this study reveals the potential of therapeutic modulation of NLK for the management of the endocrine-resistant breast cancers with active NLK signaling.
INTRODUCTION
Endocrine therapy is the most common and effective therapy for estrogen-receptor (ER)-positive breast cancers. The selective ER modulator (SERM) tamoxifen is a widely used endocrine agent, especially for premenopausal patients (1). However, many ER-positive breast tumors treated with endocrine therapy will develop endocrine resistance, especially in the advanced disease. Based on the onset of resistance during the course of endocrine treatment, endocrine resistance can be classified into de novo (primary) or acquired (secondary) resistance (2). Many ER modulators, cell cycle regulators, key growth factor receptors, and downstream kinases have been implicated in conferring endocrine resistance. To date, only a few targeted agents, especially mTOR and CDK4/6 inhibitors, have proven effective in overcoming it, and resistance eventually develops in most patients due to the emergence of alternative survival pathways (3,4). The results from recent clinical trials for the drugs targeting the known survival pathways suggest that there are likely many escape pathways that endow resistance to endocrine therapy (3,5). This fuels the need to discover key new targets in the unknown escape pathways and drugs that can block these targets.
In this study, our integrative analysis of genomic datasets identified Nemo-Like Kinase (NLK), whose overexpression is associated with worse outcome following endocrine therapy, as an actionable kinase target for endocrine-resistant breast cancer. As a member of the mitogen-activated protein kinase family, NLK encodes a serine/threonine-protein kinase that regulates a wide range of transcription factors and determines cell fate during development. In addition, NLK is known to play an important role in NGF-stimulated neurite outgrowth and stress responses (6,7). In cancer, NLK has been reported to play a crucial role in cell proliferation, invasiveness, and apoptosis through regulating a variety of transcription factors (8). However, NLK may act as either a tumor suppressor or an oncogene depending on the tumor contexts. (8–11). For example, in prostate cancer, NLK overexpression was found to inhibit androgen receptor transcriptional activity and induce apoptosis (9). In contrast, overexpression of NLK was found to promote the proliferation and invasion of lung cancer, and predicts poor prognosis (10). The function of NLK in breast cancer remains controversial. An earlier report suggested that NLK suppresses proliferation and induced apoptosis in breast cancer (12). However, a most recent report suggested that NLK predominantly localized to the nucleus of breast cancer cells, in contrast to the cytoplasmic localization in normal breast tissues, which is considered as a mechanism to protect cancer cells from apoptosis (13). To date, the function of NLK, especially the nuclear-localized protein, and its role in breast cancer endocrine resistance have not been characterized. Our study reveals a novel role of NLK in breast cancer endocrine resistance and identifies a highly selective dual p38 and NLK inhibitor, VX-702, that exhibit significant therapeutic effects when combined with mTOR inhibitors in cell line and patient-derived xenograft mouse models of primary and acquired endocrine resistance.
MATERIALS AND METHODS
Gene expression data and survival analysis
To evaluate the predictive value of NLK overexpression, we compiled several publicly available gene expression datasets from Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/), including Loi et al., (GSE6532, tamoxifen-treated and untreated) (14), Symmans et al., (GSE17705, tamoxifen-treated) (15), and Wang et al., (GSE2034, untreated) (16). To correct for batch effect, gene expression signals were normalized with quantile normalization. These processed expression signals were then applied to survival analysis. The NLK probe set “218318_s_at” that is present in both Affymetrix U133A and U133 plus2 platforms was used in the analysis. NLK overexpression was defined based on the cut off of median + MAD (median absolute deviation) calculated in R for each expression dataset using default constant (1.4826). Kaplan-Meier analyses were carried out using the R survival package. Patients were divided into two groups based on the above cut-off (NLK high and the rest). Follow-up time was constrained to a maximum of 10 years. P values were derived from the “survdiff” function of the R package implementing the log-rank test (rho=0) (uncorrected for multiple comparisons). For the Affymetrix Human Exon 1.0 ST data for breast cancer cell lines (17), exon expression signals were extracted using the RMA-sketch of Affymetrix power tools and gene expression signals were summarized by taking the mean of exon expression values.
Cell culture
BT483, MDA-MB-415, CAMA1, HCC1428, T47D, and MCF12A cells were obtained from American Type Culture Collection (ATCC). ATCC distributed cell lines are authenticated routinely through short tandem repeat (STR) profiling and are low passage and contamination free. The MCF7-TamR and T47D-TamR cells were generated in Dr. Rachel Schiff’s lab and were provided to us along with the parental MCF7 cells, as previously described (18–20). The MCF7 cells were authenticated by MD Anderson Cell Authentication Core Facility through STR profiling. HCC1428, and BT483 cells were cultured in RPMI 1640 (ATCC) with 10%−20% fetal bovine serum and 200 mg/ml L-glutamine (Invitrogen) according to ATCC recommendation. MDA-MB-415 cells were cultured in DMEM (Thermo Fisher Scientific) with 10% fetal bovine serum and 200 mg/ml L-glutamine (Invitrogen). MCF12A cells were cultured as previously described (21). Tamoxifen-resistant cells (TamR) were established by continuous passage of parental cells in the presence of 10−7 M Tam. TamR cells were propagated in phenol red-free RPMI 1640, with 5% charcoal-dextran treated fetal bovine serum (CD-FBS, Thermo Fisher Scientific) and 10−7 M Tam. Cell lines are routinely tested for mycoplasma contamination following a published protocol (13).
Plasmid construction, transfection, and infection
The NLK-specific siRNA#1 (5′-GUCAGUAACAGAUCCAAGA-3’) siRNA#2 (5’-GAGCUGAAUUUGAAGACUA-3’) and control siRNAs (ON-TARGETplus Non-targeting Pool, D-001810-10-50) were purchased from Dharmacon. Two batches of siRNA#1 from Dharmacon were used in our studies, the lot number used for Figure 1b–c and 3a is WXIFW-000005, and the lot number for Fig. 3g–h is WXIFW-000015. We also used a Sigma version NLK siRNA#1 (Supplementary Fig. S5). While all these three versions of siRNA#1 have the same sequence, the batch WXIFW-000005 showed less knockdown efficiency compared to the other two versions. The siRNAs were transfected using Lipofectamine RNAi MAX Reagent (Invitrogen) according to the manufacturer’s protocol. The full-length cDNA of NLK was purchased from Origene (Catalog #: SC124920), and the Open Reading Frame (ORF) was introduced into the inducible lentiviral pTINDLE vector (22). This vector features an inducible promoter (pTRE-tight), a transactivator (rtTA3), and a lentiviral backbone for effective gene delivery. All subclones were verified by capillary sequencing. The NLK overexpression vector used for the rescue experiment contains a few silent mutations that do not affect the amino acids. Selected cell lines were infected with the lentiviral vectors in medium containing 6 μg/ml polybrene (Sigma-Aldrich). The stable lines expressing NLK ORF were selected with Geneticin (Invitrogen). Doxycycline (Dox) (Sigma-Aldrich) was used for the induction of NLK ORF (10–2000 ng/ml Dox).
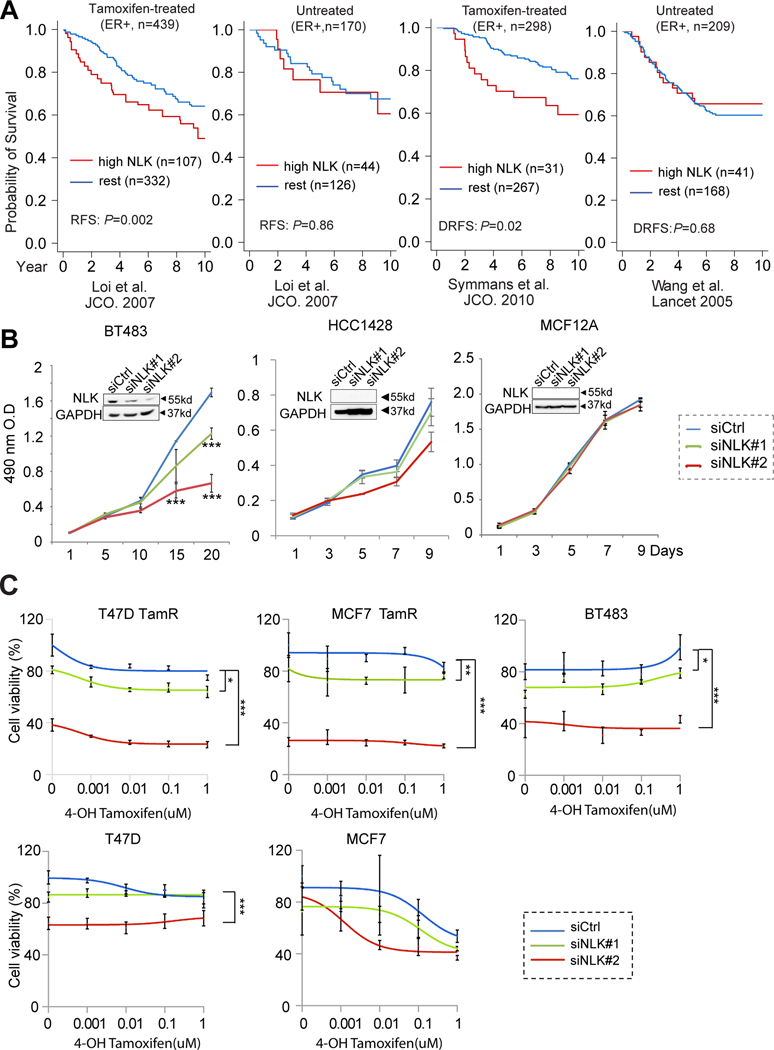
Figure 1. Identification of NLK as a new kinase target in endocrine-resistant breast cancer.
(a) NLK overexpression predicts worse outcome in ER+ breast cancer patients treated with tamoxifen monotherapy but not in untreated patients. The association of NLK overexpression with recurrence free survival in tamoxifen-treated and untreated luminal breast cancer patients were assessed using public gene expression datasets by Loi et al., (14), Symmans et al., (15), and Wang et al., (16). DRFS, distant recurrence free survival. Here the cutoff for NLK overexpression is determined based on median plus one median absolute deviation (see Methods). (b) The effect of NLK inhibition by two independent siRNAs on the growth of the BT483 and HCC1428 luminal breast cancer cell lines, as well as the MCF12A benign breast epithelial cells. (c) The therapeutic effect of NLK silencing by two independent siRNAs on 4-OH tamoxifen treatment in the primary endocrine-resistant BT483 cells and the acquired tamoxifen resistant (TamR) clones of MCF7 and T47D cell lines as well as the parental MCF7 and T47D cells. *P<0.05; **P<0.01; ***P<0.001 based on t-test (time-point experiments) or ANOVA (tamoxifen dose curves) compared to siRNA controls. The error bars represent the standard deviations.
Figure 3. NLK phosphorylates ERα and its key coactivator SRC-3 and modulates ER transcriptional activity in endocrine-resistant breast cancer cells.
(a) ERE luciferase reporter assay showing the effect of NLK inhibition/overexpression or VX-702 treatment on ER transcriptional activity in BT483 cells under estrogen deprivation (ED) and 0.5uM tamoxifen treatment. (b) VX-702 significantly inhibits the ER transcriptional activity in MCF7-TamR and T47D-TamR cells in the presence E2, or ED plus different doses of 4-OH tamoxifen treatment. a-b, *P<0.05; **P<0.01, *** P<0.001. P-value was calculated by t-test. (c) Co-immunoprecipitation of ERα using anti-ERα antibody and WB using anti-NLK antibody or anti-ERα antibody in BT483 overexpressing endogenous NLK. (d) In vitro kinase assay of recombinant active NLK using ERα as substrate with or without VX-702 treatment. Bar chart presents the quantified band intensity. The error bars in 3a-c represent the standard deviations. (e) Western blot analysis of ER signaling in BT483 cells following NLK silencing and endocrine treatment. BT483 cells were seeded in phenol red-free medium with 5% charcoal-dextran-stripped FBS containing 0.5uM Tamoxifen or vehicle (ethanol) for 48 hours, and then reverse transfected with siCtrl, siNLK#1, siNLK#2 (10nM) for 72 hours. (f) NLK phosphorylates SRC-3. Upper panel, in vitro kinase assay using recombinant NLK and SRC-3 proteins after the indicated treatment. Middle panel, in vitro kinase assay using recombinant NLK and 1–6A mutant SRC-3 protein. Lower panel, in vitro kinase assay using recombinant NLK and SRC-3 protein with mutation at indicated site (1–6A mutation sites: T24A, S505A, S543A, S857A, S860A, or S867A). (g) ER target gene expression changes following NLK inhibition significantly correlates with their changes following tamoxifen treatment in BT483 and T47D TamR cells. Left, log2 ratio of ER target differential expression (DE) following Tamoxifen treatment correlated with siNLK #1/2 treatment in BT483 cells. Here we used the ER target genes (n=76) compiled from TRUST database (70) in the analysis. Middle, log2 ratio of ER target gene DE following Tamoxifen treatment compared to siNLK #1/2 treatment in T47D TamR cells. Here T47D specific ER target genes (n=83) provided by Lin et al are used in the analysis (71). Right, log2 ratios of ER target DE between Tamoxifen and VX-702 treatment in T47D TamR cells. The T47D specific ER target genes (n=83) are used in the analysis. The Pearson correlation coefficients are shown in the figure with all p-values less than 0.001.
Cell viability assays
Cell viability was measured for 5–7 days by MTS cell proliferation assay using the CellTiter 96 kit according to the manufacturer’s directions (Promega). 4-Hydroxytamoxifen (Tam) were obtained from Sigma-Aldrich (H7904); the NLK inhibitor VX-702 was obtained from LC Laboratories (V-9366, Fig. 2D and 2E), or Vertex Pharmaceuticals Incorporated (Lot A2805–25B, Fig. 2E, 2F–G, and Fig. 3–6). For tamoxifen-sensitivity studies, cells were first deprived of estrogen (ED) using phenol red-free medium with 5% charcoal-dextran-stripped FBS for 48h. The cells (5000/well for BT483, 2500/well for the rest cell lines) were then seeded and reverse transfected with siRNA (final concentration 10 nM), and exposed to different doses of 4-Hydroxytamoxifen (0.0005–1 μM) or VX-702 (0.005–10 μM) at 48 hr post transfection. Cell viability was assessed after 7 days (all cell lines except BT483) or 14 days (BT483) following drug treatment and the surviving fraction of cells for each dose of drug was calculated by dividing the OD value from the drug-treated wells with that of the vehicle-treated wells.
Figure 2. Identification of a novel dual p38 and NLK inhibitor VX-702, and its therapeutic effect to tamoxifen treatment in vitro.
(a) Heatmap of “hot-spot” drug-kinase assay identifying VX-702 as a potent dual p38 MAPK and NLK inhibitor with exclusive activity against p38 MAPKs and NLK. The percentage of kinase activity inhibition is shown in the red color scale. (b) Bar chart showing the inhibition efficacy of VX-702 over a panel of 300 kinases based on the same dataset as in A. (c) In vitro kinase assay using recombinant NLK protein and MBP as the substrate in the presence or absence of VX-702 treatment. (d) In vitro kinase assay for V5-NLK immuno-precipitated from VX-702 treated MCF7 cells using MBP as the substrate. In order to maintain the inhibition of NLK, the immunoprecipitation product was incubated in different doses of VX-702 throughout the in vitro kinase assay process. (e) The survival fraction of MCF7-TamR breast cancer cells and MCF10A non-cancerous breast epithelial cells following treatment with different doses of VX-702 for 7 days; 0.5 μM was determined as the effective concentration in vitro. (f) The therapeutic effect of VX-702 on 4-OH tamoxifen treatment in primary and acquired tamoxifen resistant breast cancer cell lines. The assays were carried out for 7 days under estrogen deprived condition. (g) Induction of ectopic NLK expression rescues the therapeutic effect of VX-702 to tamoxifen treatment in the MCF7-TamR and T47D-TamR cells. ***P<0.001 (based on two-way ANOVA). The error bars represent the standard deviations.
Figure 6. The therapeutic effect of VX-702 as a single agent or adjuvant to everolimus in the WHIM 11J PDX model with de novo tamoxifen resistance.
(a) WHIM 11J PDX tumors were transplanted into ovariectomized female nude mice in the absence of estrogen or tamoxifen supplementation. Upon tumor establishment, mice were randomized into four treatment arms: vehicle, VX-702 (50mg/kg, o.g BID), everolimus (Eve, 5 mg/kg, o.g daily), or VX-702+everolimus. Left: the average tumor volumes of each treatment group. Right: the tumor volumes of different treatment arms on Day 22. The Boxplot illustrates the distribution of the tumor volumes of each treatment group based on the following: minimum, first quartile, median, third quartile, the maximum, and the outliers. The error bars represent the standard deviations. (b) Kaplan-Meier survival plot comparing the progression-free survival of different treatment arms. Progression-free survival was analyzed based on tumor-tripling time. (c) The body weight measurements of mice in different treatment arms. *P<0.05; **P<0.01; *** P<0.001. P-values were calculated based on two-way mixed ANOVA for comparing the tumor volumes and generalized Wilcoxon test for progression-free survival.
Western blot
To extract total proteins, cells were homogenized in RIPA Lysis Buffer (Sigma-Aldrich), supplemented with complete protease inhibitor cocktail tablet (Roche Diagnostics). Thirty micrograms of protein extracts were denatured in sample buffer, separated by SDS-PAGE, and transferred onto a nitrocellulose membrane (Invitrogen). The membranes were blocked for one hour and incubated overnight at 4°C with rabbit polyclonal anti-NLK (1:1000, Sigma, #N8288), or with rabbit polyclonal anti-beta actin (1:1000, Cell Signaling Technology, #4970) antibodies. Membranes were then incubated with horseradish peroxidase-conjugated secondary antibody and the signals were visualized by the enhanced chemiluminescence system (Thermo Fisher Scientific) per the manufacturer’s instructions. Polyclonal antibodies against cyclin D1 and ERα were purchased from Santa Cruz Biotechnologies and Thermo Fisher, respectively. pERα-S118 (2511) antibody was purchased from Cell Signaling Technology, and pERα-S104/106 (BS3131R) and pERα-S167 (BS0894R) were purchased from Bioss ANTIBODIES.
Luciferase assay
Cells were co-transfected with 1 μg of an ERE (estrogen transcriptional response element) luciferase reporter construct (ERE-TK-Luc) and 0.1 μg of pCMV β-galactosidase as an internal control for transfection efficiency in serum-free medium using XtremeGene HP (Roche Diagnostics). Such vector normalization is recommended for transiently transfected cells (23). After 24h, the cells were treated with or without doxycycline for 72 h followed by E2 or Tam treatment for another 24h. The luciferase levels were then measured with a Luciferase Reporter Assay kit (Promega) in a luminometer and normalized to β-galactosidase activity.
Co-Immunoprecipitation Assay
To study NLK/ER complex formations, monoclonal antibodies against V5 and ER-alpha (Thermo Scientific), and protein A/G–sepharose beads (Santa Cruz) were used. Fresh protein lysates in Tween-20 Co-IP buffer (1000 μg) were immunoprecipitated overnight at 4°C with constant rotation. After washing 3 times with the extraction buffer, the co-immunoprecipitated proteins were analyzed by Western blot as described previously. Whole cell protein lysate (50 μg) was loaded as a control input. The immunoprecipitation assay was repeated 3 times with identical results.
In vitro kinase assay
0.5 μg of Full length ERα Recombinant Protein (Life Technologies, RP-310) or 0.5 μg of recombinant SRC-3 protein (a gift from Dr. Bert W. O’Malley) (24) was incubated with 0.1 μg of active recombinant full-length NLK protein N15–10G (SignalChem) in the kinase buffer (SignalChem; 5mM MOPS, 2.5mM beta-glycerol-phosphate, pH 7.2, 5 mM MgCl2, 1 mM EGTA, 0.4 mM EDTA and 0.25 mM DTT) containing 50 μM ATP and 2 μCi [32P]γATP at 30 °C for 30 minutes. To examine whether VX-702 inhibits NLK activity in phosphorylating ER, VX-702 was added in the reaction mixture described above and then incubated at 30 °C for 30 minutes. NLK kinase activity towards ER or SRC-3 was then analyzed by SDS-PAGE followed by autoradiography.
In vivo xenograft models and preclinical studies
All in vivo experiments using mice were performed in accordance with a protocol approved by the Baylor College of Medicine Institutional Animal Care and Use Committee (IACUC). All mice used in this study were ovariectomized. Briefly, T47D-TamR (#156L) tumor fragments (~1mm3) were implanted subcutaneously (s.c.) into 6–8 week-old female athymic nude-Foxn1nu mice (Harlan Sprague Dawley Inc.) supplemented with tamoxifen (Sigma #T9262). Tamoxifen is dissolved in peanut oil then injected subcutaneously. Tumor growth was monitored daily and measured three times a week using digital calipers. Tumor volume was calculated based on the formula (V = L × W × W × 0.5). When tumors reached the size of 150–200 mm3, tamoxifen was withdrawn and mice were randomized into six treatment groups (vehicle, VX-702, Ful, Ful+VX-702, everolimus, everolimus+VX-702). VX-702 (provided by Vertex Pharmaceuticals Incorporated) was administered twice daily at 20 mg/kg for the pilot study, either alone or in combination with Ful. Thereafter VX-702 was given at 50mg/kg BID via oral gavage in the subsequent studies. Everolimus (LC labs #E-4040) was administered daily at 5 mg/kg by oral gavage. Tamoxifen was administered subcutaneously at 500 μg/mouse, 5 days/week. Ful (Selleckchem #S1191) was administered subcutaneously at 5 mg/mouse once per week. Mice were euthanized when the tumor size reached the upper limit of 1500 mm3 or after 2 weeks of treatment for biomarker and RPPA analysis. Tumor tissues were snap frozen for biochemical analysis or FFPE fixed for pathological analysis. The WHIM 11J PDX line was provided by Dr. Shunqiang Li of the Human & Mouse Linked Evaluation of Tumors (HAMLET) Core at Washington University. The WHIM 11J single cell solution was mixed with Matrigel (BD) and s.c injected bilaterally into 4–6 week old ovariectomized athymic nude mice (n=4) to establish the WUSTL PDX generation 1 (G1) model without estrogen or tamoxifen supplementation. The biggest G1 tumor was collected and dissected into small tumor fragments, then implanted into 5 mice to obtain G2 WUSTL PDX models. Established G2 tumors were serially expanded to 80 mice, 60 of which were randomized into one of four treatment groups: vehicle, VX-702 (50 mg/kg, o.g., BID), everolimus (5 mg/kg, o.g. daily), or VX-702+everolimus (15 mice per group). The rest 20 mice bearing the G2 tumor were randomly distributed into one of four treatment groups as above but with tamoxifen supplementation: tamoxifen (500 μg/mouse), tamoxifen+VX-702, tamoxifen+everolimus, tamoxifen+everolimus+VX-702. The drugs were administered as described above. Mice were euthanized when the tumors reached 1500 mm3 or at study endpoint, and the tumor tissues were snap frozen or FFPE fixed as described above.
Statistical analysis
P-values of the experimental results were analyzed by unpaired Student’s t-tests (for other experiments) or ANOVA tests (for drug dose curves), and all data are shown as mean ± standard deviation. Analysis of the xenograft experiments focused on the comparison between different treatments. For the in vivo study, statistical comparisons of tumor growth rates were performed using two-way mixed ANOVA that takes account of mice groups and time points as factors, and mouse subjects as random effects (25–27). Long-term outcomes were evaluated by survival analysis methods. ‘Events’ were defined to mimic clinically relevant outcomes; time to tumor progression (tumor-volume-tripling) was analyzed using Kaplan-Meier survival curves and compared by the generalized Wilcoxon test.
Additional methods are provided in Supplementary Methods.
RESULTS
Identification of NLK as a kinase target associated with worse outcome in tamoxifen-treated patients
To systematically reveal the new therapeutic targets in breast cancer, we used genomic data from TCGA to identify the genes that are upregulated by recurrent amplifications in ER+ breast cancer genomes (>2%) (28). Druggable genes were then selected according to a drug-target database compiled from multiple sources (22), which were then benchmarked by the cancer ConSig score to prioritize functionally important genes underlying cancer(29,30) (see Methods). This analysis revealed many known amplified kinases, such as ERBB2, PTK2, PAK1, RPSKB1, and TLK2(22), as well as a novel kinase target, NLK (Supplementary Table S1). Copy-number data revealed that NLK is targeted by focal genomic amplifications in about 3% of breast tumors (Supplementary Fig. S1A), which correlates with NLK overexpression (Supplementary Fig. S1B). This suggests NLK amplification as a rare genetic event to lock in NLK overexpression in some patients which implies potential cancer cell addiction. Further analysis of gene profiling data revealed overexpression of NLK in 19.6% of breast cancers. The frequency of NLK overexpression is markedly higher in the more aggressive and endocrine-resistant luminal B subtype, compared to luminal A tumors (P=0.0003) (Supplementary Fig. S1C).
Since luminal B tumors have a higher risk of early relapse with endocrine therapy (31), we assessed the correlation of NLK expression with the outcome of tamoxifen treatment in breast cancer patients using public gene expression datasets matched with patient survival data (14–16). In these datasets, recurrence free survival (RFS) and/or distant relapse free survival (DRFS, also known as distant metastasis free survival or DMFS) are used as endpoint to evaluate patient outcome. These endpoints are commonly used in clinical trials for luminal breast cancer patients as they are not affected by deaths from competing causes and additional second line or palliative therapies that can bias the treatment outcome comparison, and is particularly advantageous when there are lengthy intervals between recurrence and death (32). We first analyzed an established dataset by Loi et al. (14) (Fig. 1A) with well-annotated survival data for ER+ patients treated with tamoxifen monotherapy. In the 439 ER+ patients treated with adjuvant tamoxifen monotherapy, those with tumors overexpressing NLK showed significantly worse RFS (P=0.002). Next, we assessed the correlation of NLK expression with the excess relapse rates in the tamoxifen treated patients stratified into luminal A or B groups. The patients with high excess relapse rates after adjuvant tamoxifen treatment represent individuals that relapse too soon (33), thus can be considered endocrine-resistant. As shown in Supplementary Fig. S2A–B, the increased excess relapse rates positively correlate with the level of NLK overexpression in both luminal A and B patient groups. In addition, NLK overexpression also correlates with worse DMFS in tamoxifen treated patients (Supplementary Fig. S2C). This result was further verified in an independent dataset of tamoxifen-treated patients (Symmans et al., P=0.02) (15) (Fig. 1A). In contrast, NLK expression did not significantly affect the RFS in the absence of endocrine treatment in the Loi et al. dataset (n=170, P=0.86) or DRFS in the Wang et al., dataset (n=209, P=0.68) (16). These data suggest the specific association of NLK overexpression with worse clinical outcome in tamoxifen-treated patients and reveal the potential role of NLK in breast cancer endocrine resistance.
The effect of NLK silencing in luminal breast cell lines with reduced endocrine sensitivity or acquired endocrine resistance
Next, we examined NLK protein in a panel of ER positive and Her2 negative breast cancer cell lines, as well as the tamoxifen resistant (TamR) clones derived from MCF7 and T47D cells (Supplementary Fig. S3). Among these cell lines, NLK overexpression was detected in BT483, MDAMB415, and CAMA1 cells, and to a lesser degree in ZR-75–1 cells. Among these, BT483, MDAMB415, and ZR-75–1 are known to exhibit reduced endocrine sensitivity (20,34,35). NLK expression was also upregulated in the T47D TamR clone and modestly in the MCF7 TamR clone compared to their parental cells. To assess the therapeutic role of NLK in endocrine-resistance, we designed two independent siRNAs #1 and #2 (Dharmacon) targeting the ORF or 3’ UTR, respectively. We first assessed the specific effects of these siRNAs against NLK using the NLK-overexpressing BT483 cells, and two cell lines with undetectable NLK levels, including the HCC1428 breast cancer cell line, and the MCF12A benign breast epithelial cell line (Fig. 1B). The growth of BT483 cells was significantly inhibited by both the siRNAs. The inhibitory effect was more pronounced with siRNA #2, consistent with its more potent effect on inhibiting NLK protein levels as shown by western blots (Fig. 1B). In contrast, the growth of the negative control cell line HCC1428 and MCF12A was not significantly affected by these siRNAs. To further verify the specificity of the siRNA against NLK, we performed knockdown and rescue experiments using BT483 cells engineered to overexpress a doxycycline-inducible NLK open reading frame (ORF), which is immune to siRNA #2 since it targets the 3’UTR region of NLK. MTS cell proliferation assay showed that induction of NLK overexpression with doxycycline in BT483 cells significantly rescued the growth inhibitory effect of siRNA #2, further supporting the specificity of this siRNA against NLK (Supplementary Fig. S4).
Next, we assessed the effect of NLK inhibition on the tamoxifen response of BT483 cells known to exhibit reduced tamoxifen sensitivity (34), and the MCF7 and T47D clones with acquired tamoxifen resistance (TamR) together with their parental cells. As a vast majority of breast cancer patients are postmenopausal, and recent clinical trial suggested that the combination of two year-ovarian suppression to tamoxifen improves survival in premenopausal women, testing the effect of tamoxifen in the absence of estrogen could provide more clinically relevant results. We thus cultured the cells in estrogen-deprived (ED) condition and then treated them with increasing doses of 4-hydroxytamoxifen (4OHT) (0.001–1μM) together with NLK siRNAs or control siRNA. 4-OHT is commonly used for in vitro studies to ensure the bioavailability of the active metabolite. NLK silencing significantly inhibits the viability of the breast cancer cells treated with tamoxifen under estrogen deprivation (ED), with more obvious effects in the acquired endocrine-resistant T47D and MCF7 clones compared to their parental cells. In addition, the effects are more significant with siRNA #2 than siRNA #1 due to better inhibition of NLK by this siRNA, as described earlier (Fig. 1C). It is notable that under ED condition, knockdown of NLK impacted cell viability independent of whether 4-OHT is present. The effect of NLK silencing independent of tamoxifen could be attributed to its repression of ER transcriptional activity as discussed later.
In the above experiments, siRNA#1 yielded relatively modest inhibition of NLK, which we found appeared to be related to the batch effect of this reagent. We also tested another version of siRNA#1 we obtained from Sigma that share the same sequence as the Dharmacon version but exhibits more potent inhibition of NLK expression (Supplementary Fig. S5A–B). We then assessed the effect of this siRNA on the endocrine responses of BT483, MDAMB415, and CAMA1 cells, as well as MCF7 and T47D Tam-R cells (Supplementary Fig. S5C). CAMA1 cells are known to be endocrine-sensitive (36), which showed better response to tamoxifen treatment than other models in our experiment, although the responsiveness appears to be dampened from estrogen deprivation prior to the treatment. Consistent with the more potent NLK silencing effect of this version siRNA#1, MTS assay revealed a more profound inhibition of cell viability and additive effect to tamoxifen treatment by this siRNA in the NLK-overexpressing cell line models treated with estrogen deprivation and 4-OHT. Collectively, these data suggest the potential therapeutic role of NLK in breast cancer endocrine resistance.
Identification of a selective dual p38 and NLK inhibitor, VX-702
To discover novel NLK inhibitors, we investigated the public drug-target and kinase inhibitor profiling datasets (37–41). Surprisingly, several p38 MAPK inhibitors were found to possess strong activities against NLK (Fig. 2A). This may be attributable to the homology between NLK and p38 MAPK in the kinase catalytic domain (Supplementary Fig. S6). In addition, both NLK and p38 belong to the same subfamily that also include ERK and JNK(39). Among those p38 inhibitors, VX-702 showed an exclusive inhibitory effect against p38 and NLK (Fig. 2B). VX-702 is a highly selective inhibitor of p38 that competitively binds to the ATP pocket of the kinase catalytic domain. This drug has been tested in phase I-II clinical trials for treating inflammatory diseases (42). Kinase profiling data (43) suggest that, at 0.5μM, VX-702 inhibits 94.7% of p38α and 77.7% of p38β activities, and 84.2% of NLK activity. In contrast, the maximum inhibition of other 297 kinases are 37.2% (the inhibition of ERK1 and ERK2 are 4.8% and 14.3% respectively, and the inhibition of JNK1, JNK2, and JNK3 are 0%, 6.5%, and 1.6 respectively). This suggests VX-702 as a highly selective inhibitor against p38 and NLK. To verify the activity of VX-702 against NLK, we performed in vitro kinase assay using recombinant active NLK protein (SignalChem) with Myelin Basic Protein (MBP) as the substrate (Fig. 2C). In addition, we also isolated the ectopically expressed V5-tagged NLK protein, incubated it with different doses of VX-702, and performed an in vitro kinase assay (Fig. 2D). As a result, we observed auto-phosphorylation of the NLK protein itself, in addition to the NLK-mediated phosphorylation of MBP. This is consistent with the previous report that NLK auto-phosphorylates itself (44). Importantly, our experiments showed that VX-702 potently inhibited the NLK-mediated phosphorylation of MBP as well as NLK auto-phosphorylation, thus verifying its activity against NLK (Fig. 2C–D).
VX-702 exhibits therapeutic effects in endocrine-resistant breast cancer cells
To determine the effective therapeutic dose of VX-702, we tested the effects of different doses of VX-702 on the growth of MCF7-TamR breast cancer cells and MCF10A breast epithelial cells, which revealed an optimal therapeutic dose of 0.5μM (Fig. 2e). We then treated the primary endocrine resistant BT-483 and MDA-MB415 cells as well as the acquired MCF7 and T47D tamoxifen-resistant clones with 0.5μM VX-702 and titrated 4-OH tamoxifen under ED condition. VX-702 exhibited significant therapeutic effects in all these resistant lines (Fig. 2F). Next, to validate that the therapeutic effect of the dual P38 and NLK inhibitor VX-702 was through inhibition of NLK, we assessed the impact of NLK or p38 knockdown on the endocrine responsiveness of these cells. According to literature, p38α is the most abundant p38 isoform in human breast tumors (45), and has been suggested to promote endocrine resistance (46), while p38β is not expressed in breast cancer cells. We thus performed siRNA knockdown of NLK or p38α, either alone or together in the BT483, MCF7-TamR, and T47D-TamR cells. While only a modest inhibitory effect was observed upon p38 silencing, under ED condition, knockdown of NLK potently inhibited the cell growth, with no further inhibition observed by simultaneous silencing of NLK and p38 (Supplementary Fig. S7). To further attribute the inhibitory effects of VX-702 to its activity against NLK, we engineered the MCF7-TamR and T47D-TamR cells to inducibly overexpress NLK, and treated the cells with 0.5μM VX-702 and titrated tamoxifen doses. NLK overexpression rescued the therapeutic effect of VX-702 treatment to tamoxifen (Fig. 2G). Together, these findings support the rationale of using VX-702 to target NLK for the treatment of endocrine-resistant breast cancer.
NLK phosphorylates ERα and its key coactivator SRC-3 to modulate ER transcriptional activity
Previous studies suggested that NLK localizes to the nucleus when activated and regulates a wide range of transcriptional factors (44). NLK is also known to inhibit androgen receptor signaling in prostate cancer (9). We thus speculate that NLK may modulate ER transcriptional activity in the context of tamoxifen resistance. To test this, we first evaluated the impact of NLK knockdown or overexpression on the activity of the estrogen responsive elements (ERE) in endocrine-resistant breast cancer cells using an ERE luciferase reporter construct (ERE-TK-Luc), and pCMV β-galactosidase as an internal control as previously reported (47–49). In BT483 cells, under ED and 0.5μM 4OHT treatment, NLK silencing by the two siRNAs significantly decreased the ERE activity (Fig. 3A, left), while ectopic overexpression of NLK increased this activity (Fig. 3A, middle). Next, we assayed the effect of VX-702 on the ERE activity of the BT483, MCF7-TamR and T47D-TamR cells. Decreased ER transcriptional activity in response to exogenous E2 or to the agonistic activity of Tam under ED condition was observed in all three cell lines following VX-702 treatment (Fig. 3A right, and 3B). Together, these data suggest the role of NLK in modulating the ER transcriptional activity and the potential of VX-702 in blocking it.
Phosphorylation of ERα by different kinases has been reported as a key mechanism of ERα transcriptional activation in tamoxifen-resistant breast cancer (50). We thus examined whether NLK directly regulates and phosphorylates ERα. First, to test if the NLK protein physically interacts with ERα, we immunoisolated ERα in the BT483 cells and then detected coprecipitation of NLK protein. Subsequent western blot showed that NLK co-precipitates with ERα (Fig. 3C). This result was further verified by a reverse immunoprecipitation (IP) via isolating the ERα protein complex and probing with ERα antibody in MCF7 cells transduced with V5-tagged NLK (Supplementary Fig. S8). To further examine if NLK directly phosphorylates ERα, we performed an NLK kinase assay using recombinant ERα protein as the substrate. NLK protein significantly enhanced the phosphorylation of ERα (Fig. 3D), and the effect was diminished by the NLK kinase inhibitor VX-702. To assess if NLK modulates ER phosphorylations at the transcription activation function-1 (AF-1) domain, we performed western blots following NLK silencing in the BT483 cells under endocrine treatment. Under estrogen deprived condition, NLK silencing led to decreased levels of both total ER and phospho-ER (at Ser 104/106, 118, and 167) in the presence or absence of tamoxifen treatment (Figure 3E), consistent with its tamoxifen independent effect observed in our previous experiments. To examine if NLK modulate E2-induced ER phosphorylation, we treated the endocrine-responsive T47D cells with NLK siRNAs under estrogen-deprived condition and then exposed the cells to E2 briefly. As a result, NLK silencing leads to attenuation of E2-induced ER phosphorylation at Ser 104/106, 118, and 167 suggesting its function in modulating ER activity in endocrine-responsive breast cancer cells (Supplementary Fig. S9).
Since survival kinases, such as the NLK homologs p38 and ERK, often show dual regulation of ERα and the key ERα co-activator SRC-3 (46,51), we speculated that NLK might affect SRC-3 activity as well. To test whether NLK directly phosphorylates SRC-3, we performed an NLK kinase assay using recombinant active NLK protein and recombinant SRC-3 protein as the substrate. Interestingly, we also observed strong phosphorylation of SRC-3 by NLK kinase (Fig. 3F, upper panel). To identify the specific SRC-3 sites phosphorylated by NLK, we performed an in vitro NLK kinase assay using recombinant SRC-3 1–6A protein, which is engineered to harbor mutations at six major phosphorylation sites (T24A, S505A, S543A, S857A, S860A and S867A). The introduction of 1–6A mutations in the SRC-3 protein attenuated its phosphorylation by NLK, compared to the wild-type SRC-3, suggesting the role of NLK in phosphorylating at least some of these sites (Fig. 3F, middle panel). Nonetheless we cannot rule out the possibility that sites other than these six can be phosphorylated by NLK. To determine the individual SRC-3 sites that may be phosphorylated by NLK, we performed NLK kinase assay using the recombinant SRC-3 proteins with each of these individual sites mutated. Among these sites, mutation of S505A most attenuated the phosphorylation of SRC-3 by NLK, and to a lesser degree S857A and S860A (Fig. 3F, lower panel). S505 is known to be phosphorylated by p38 (52), and this site has been found to be important for ER transactivation by SRC-3 and tamoxifen resistance (53). These data suggest that NLK may regulate ER transcriptional activity and endocrine resistance via modulating both ERα and its key coactivator SRC-3.
To assess if NLK modulates ER target gene expression, we performed transcriptome sequencing following NLK inhibition or tamoxifen treatment in the BT483 or T47D Tam R cells under estrogen-deprived condition. The expression changes of ER target genes following NLK inhibition are compared to their changes following tamoxifen treatment. Our results show that the ER target gene expression changes following NLK silencing or VX-702 treatment significantly correlate with their expression changes following tamoxifen treatment (Fig. 3G). This suggests that NLK signaling support ligand-independent activity of ER, and NLK inhibition alone reduces ER transcriptional activity in the absence of tamoxifen. This tamoxifen-independent mechanism may contribute to the basal growth suppressing effect observed following NLK inhibition under estrogen deprivation.
Preclinical evaluation of VX-702 treatment in an acquired tamoxifen-resistant tumor model
Next, we sought to determine the therapeutic value of VX-702 in a xenograft model of acquired tamoxifen resistance in vivo. Pharmacokinetics data suggest that VX-702 relies on renal clearance, and both its maximum serum concentration and total drug exposure are proportional to dosage (42). According to literature, up to 50 mg/kg of VX-702 has been tested in mice (54). Among the ER+/HER2- luminal breast cancer cells, MCF7 and T47D cells are capable of forming xenograft tumors, from which we have established transplantable acquired tamoxifen-resistant (TamR) tumor lines through long-term tamoxifen treatment and serial transplantation in vivo (19,20,55). To select the appropriate tumor model, we screened these TamR tumors and their parental tumors for NLK protein expression and nuclear localization by NLK immunohistochemistry (IHC) using a documented IHC antibody (56). MCF7 and T47D cells tend to lose NLK expression and nuclear localization when grown in mice. However, after long-term treatment with tamoxifen, NLK is often upregulated (Supplementary Fig. S10). In particular, one of the T47D-TamR tumors (#156L) showed strong nuclear staining of NLK suggesting hyperactive NLK signaling. We thus re-transplanted this tumor line and tested the therapeutic effect of VX-702. The T47D-TamR xenograft tumors were established in the presence of tamoxifen, which was withdrawn during VX-702 treatment. The tumor-bearing mice were randomized into VX-702 or vehicle treatment groups. VX-702 was administered at 20 mg/kg or 50 mg/kg twice daily via oral gavage as previously described (57). At 20 mg/kg, the T47D-TamR tumors did not show any response to VX-702 treatment (Supplementary Fig. S11A). At 50mg/kg, the T47D-TamR tumors showed diverse responses to VX-702 treatment, ranging from no response to complete tumor remission (Supplementary Fig. S11B), without any significant toxicity (Fig. 4A).
Figure 4. The therapeutic effect of VX-702 treatment or in combination with mTOR inhibitor in an acquired tamoxifen-resistant tumor model derived from T47D cells.
(a) The effect of VX-702 treatment on the body weight measurements of mice from different treatment groups. NLK-high T47D tamoxifen-resistant tumors derived from #156L were transplanted into ovariectomized female nude mice and grown with tamoxifen. Upon tumor establishment, tamoxifen was withdrawn, and mice were randomized into six treatment arms: vehicle, VX-702 (50 mg/kg, o.g, BID), fulvestrant (Ful, 5 mg/mouse, s.c weekly), VX-702+Ful, everolimus (Eve, 5 mg/kg, o.g daily), or VX-702+everolimus. (b) The tumor growth curves for different treatment groups from the same in vivo experiment as in (a). Upper panel: the average tumor volumes of each treatment arm at different day points. The error bars represent the standard deviations. Lower panel: the tumor volumes of different treatment arms on Day 22. The Boxplot illustrates the distribution of the tumor volumes of each treatment group based on the following: minimum, first quartile, median, third quartile, the maximum, and the outliers. (c) VX-702 treated tumors collected at the endpoint were subjected to RPPA analysis. Tumors were sorted based on their endpoint volumes and were subdivided into two groups: large (volume >1000mm3) vs small (volume <1000mm3). Proteins that significantly differ between the two groups were plotted in a heat-map with the corresponding tumor volume for reference. The P-values are based on t-test. (d) Kaplan-Meier survival plot comparing the progression-free survival of different treatment arms. Progression-free survival was analyzed based on tumor-tripling time. *P<0.05; **P<0.01; *** P<0.001. “ns”, not significant. P-values were calculated based on two-way mixed ANOVA for comparing the tumor volumes and generalized Wilcoxon test for progression-free survival.
Next, we assessed the efficacy of VX-702 in combination with a more potent ER antagonist, fulvestrant (Ful), on these tumors. Fulvestrant works by competitively binding to ER monomers, inhibiting receptor dimerization, and accelerating ER degradation, and is the treatment option for endocrine-resistant tumors including tamoxifen-resistant tumors (58,59). The tumor-bearing mice were randomized into 4 treatment groups: Tam withdrawal, Tam withdrawal plus VX-702, Tam withdrawal plus Ful (5 mg/mouse, s.c, once per week), Tam withdrawal plus Ful and VX-702. Our results showed that Fulvestrant treatment, either alone or in combination with VX-702, yielded only modest attenuation of tumor growth, with the inhibitory effect being relatively stronger and significant in the combination group (Fig. 4B, Supplementary Fig. S11B).
To explore the underlying oncogenic signaling that may facilitate tumor survival in the presence of VX-702, we profiled the residual tumors following VX-702 mono-treatment by reverse phase protein array (RPPA). Interestingly, this revealed enhanced phosphorylation of mTOR at S2448, and upregulation of Notch1 and FGFR1 in the VX-702 resistant tumors (Fig. 4C). mTOR, a key regulator of cell growth and metabolism, is activated via phosphorylation at S2448 by the PI3kinase/Akt signaling pathway (60), and is known to promote endocrine resistance. Inhibitors of mTOR such as everolimus have been one of the most successful targeted agents for endocrine-resistant breast cancer (61). It is possible that in the in vivo context, cancer cells are in contact with the host cells and extracellular matrices, which could lead to activation of mTOR signaling and thus reduction of VX-702 effectiveness. This suggests the potential benefit of combining VX-702 with the mTOR inhibitor.
Dual administration of VX-702 and everolimus attenuates oncogenic signaling
We thus assessed the therapeutic effect of concomitant VX-702 and everolimus treatment in the T47D-TamR tumors. Co-administering VX-702 with everolimus resulted in significantly decreased tumor burden compared to Everolimus (p=0.0008) or VX-702 alone (p=0.03), leading to prolonged progression-free survival (p=0.009 compared to Everolimus, or p=0.076 compared to VX-702) (Fig. 4B,D, Supplementary Fig. S11B). No significant weight loss was observed following VX-702 treatment, either alone or in combination with everolimus, supporting the safety of this drug (Fig. 4A). To profile the cell signaling changes after dual administration of VX-702 and everolimus compared to either monotherapies, xenograft tumors harvested 15 days after treatments (Supplementary Fig. S12) were profiled by RPPA analyses (Fig. 5A). In addition, we also identified the proteins altered in both VX-702 and VX-702 plus Everolimus treatment arms which are likely affected by VX-702 (Fig. 5B). Likewise, we identified the genes affected by Everolimus by comparing Everolimus and Everolimus plus VX-702 arms with the rest treatment arms (Fig. 5C). Interestingly, while there were unique signaling changes observed after VX-702 and everolimus monotherapy, many key signaling molecules of breast cancer endocrine resistance such as total PI3K p110a/p85, STAT3/5a/6, SRC-3, cyclin D1, and the proliferation biomarker Ki67 were repressed only when the two targeted agents were combined (Fig. 5A). Phosphorylation of mTOR at S2448 was also significantly lower in the combination treatment group, compared to VX-702 or everolimus alone. These data support the therapeutic benefits of combining VX-702 with everolimus in treating acquired tamoxifen-resistant breast tumors that harbor active NLK and mTOR signaling.
Figure 5. Reverse phase protein array analysis of T47D-TamR xenograft tumors harvested following 15 days of treatments.
(a) T47D-TamR xenograft tumors were harvested after 15 days of treatments and then subjected to RPPA analysis. The proteins that show a trend of altered expression or phosphorylation levels (P<0.1) following concomitant everolimus and VX-702 (Eve+VX) treatment compared to everolimus or VX-702 monotreatment was plotted in the heat-map. Each column represents a xenograft tumor sample, and each row represents an antibody against a specific protein or a phosphorylation site. (b) Heat-map of proteins that show a trend of altered expression or phosphorylation levels after VX-702 alone or VX-702+everolimus treatment compared to vehicle (P<0.1). (c) Heat-map of proteins that are changed (P<0.1) after everolimus alone or everolimus+VX-702 treatment compared to vehicle.
Identifying and establishing the de novo endocrine-resistant WHIM 11J patient-derived xenograft model with nuclear localized NLK protein
Next, we sought to assess the effect of concomitant VX-702 and mTOR inhibitor treatment in a patient derived xenograft (PDX) tumor model with de novo endocrine resistance. Here we leveraged the Washington University Human-In-Mouse (WHIM) collection that has established about 10 ER+/HER2- PDX tumor lines, among which about 70–80% are de novo endocrine-resistant (62). This provided a great opportunity to further evaluate the therapeutic effect of VX-702 in the management of de novo endocrine resistance. We thus performed NLK IHC assay on a tissue microarray containing these WHIM PDX tumors, which revealed two ER+/HER2- tumors (WHIM 11J and WHIM 9J) with strong NLK nuclear staining. Both WHIM 11 and WHIM 9 have wild-type ESR1 but are de novo endocrine-resistant showing estradiol-independent growth (62). In particular, the WHIM 11J line was derived from a ER+ HER2- patient with a fulminant clinical course and little evidence of endocrine sensitivity (62). The WHIM 11J line also showed the strongest NLK nuclear enrichment among all PDX models (Supplementary Fig. S13A). Based on the RPPA data for these tumors, the WHIM 11J line showed high levels of pS70S6K and 4EBP1 protein phosphorylation, indicating active mTOR signaling (62). Thus, this model was found to be ideal to further study the therapeutic value of VX-702 in combination with the mTOR inhibitor. We thus obtained the WHIM 11J xenograft tumor specimen from the HAMLET core and successfully established the WHIM 11J tumors in nude mice in the absence of estrogen (E2) supplementation. The fastest growing tumors were used for subsequent therapeutic studies (Supplementary Fig. S13B). Furthermore, western blot and immunohistochemistry revealed strong expression of ERα protein in these tumors, suggesting that the endocrine resistance of these tumors is not related to the loss of ER expression (Supplementary Fig. S13C).
Therapeutic efficacy of VX-702 in combination with everolimus in WHIM 11J tumors
To further study the therapeutic value of VX-702 in combination with the mTOR inhibitor in de novo endocrine resistance, we first carried out a pilot experiment in which the WHIM 11J tumors were serially passaged in vivo with supplementation of tamoxifen. When the tumors reached ~200 mm3, mice were randomized, in the presence of continued tamoxifen, to four treatment groups (n=4): (I) vehicle; (II) VX-702 alone; (III) everolimus alone; or (IV) VX-702 plus everolimus. The tumor growth curves suggest that VX-702 plus everolimus combination treatment in the presence of tamoxifen resulted in only a modest but non-significant tumor growth inhibition (Supplementary Fig. S14). We hypothesized that this regimen may be more effective in the presence of a different endocrine regimen such as estrogen deprivation. We thus performed a second experiment using the WHIM 11J tumors serially passaged under the estrogen deprived condition. Ovariectomized nude mice bearing tumors of ~200mm3 size were randomized to four treatment groups (n=14–16), as described above, under continued ED. After 3 weeks of respective treatments, the xenografts treated with vehicle showed continued aggressive growth. While treatment with VX-702 or everolimus alone resulted in modest non-significant tumor growth attenuations, the concomitant treatment with VX-702 and everolimus showed stronger and significant growth inhibitory effect, compared to either vehicle or each drug alone, resulting in prolonged progression free survival (Fig. 6, Supplementary Fig. S15). It is worth mentioning that several WHIM 11J derived tumors displayed either attenuated growth or even regression following VX-702 or everolimus mono-treatment (Supplementary Fig. S15), which may represent the tumor subpopulations that is more sensitive to anti-NLK or mTOR monotherapy. Together, our data suggest that the combination of the dual p38/NLK inhibitor VX-702 and the mTOR inhibitor everolimus may be beneficial in treating de novo endocrine-resistant tumors harboring active NLK and mTOR signaling.
DISCUSSION
Endocrine resistance in breast cancer treatment is a critical clinical problem. The mechanisms of endocrine resistance are complex and multifaceted. The potential significance of this study can be summarized in two-fold. First, it reveals NLK as a novel mediator and kinase target for breast cancer endocrine resistance. Second, it identifies a small molecule dual p38 and NLK inhibitor VX-702 that could potentially improve the clinical management of endocrine-resistant breast cancer, especially when used concomitantly with existing therapies. NLK has been suggested to function as a tumor suppressor in breast cancer by recent studies (12,13). Until now, the role of NLK signaling in the ER pathway and endocrine-resistant breast cancer is ill-understood. Although NLK may be pro-apoptotic in certain cell contexts, our data show that NLK inhibition appears to be therapeutic in the endocrine resistance setting. It is possible that NLK may play a multifaceted role in breast cancer cells and the cellular context following endocrine therapy stress may have converted the function of NLK. In fact, in breast cancer, such multifaceted functions are also observed for other targets such as p38, JNK, and TGFβ, which are generally considered as tumor suppressors but become oncogenes when the breast cancer cells develop endocrine resistance or metastasis (63–66).
Our subsequent mechanistic studies revealed that NLK may endow endocrine resistance, at least in part, via enhancing the phosphorylation of ERα itself and its key coactivator SRC-3 to modulate ER transcriptional activity under endocrine stress. Similarly, the stress kinase p38, the homolog of NLK, has been reported to modulate ER function and promote endocrine resistance through phosphorylating both ER and SRC-3 (5,67–69). Future investigations will be required to elucidate the precise NLK phosphorylation sites on ER and SRC-3 and their respective functions.
Through investigation of a kinase inhibitor profiling dataset (41), we have identified VX-702, a highly selective dual p38 and NLK inhibitor, presumably due to their homology in the kinase domain (Supplementary Fig. S6). This drug was originally developed as a p38 inhibitor, but its activity against NLK was just revealed by our analysis of a kinase profiling dataset for commercially available kinase inhibitors against a panel of recombinant protein kinases (41). The present data show that VX-702 represses ER transcriptional activity and presents therapeutic effect in breast cancer cell lines with either de novo or acquired endocrine resistance to tamoxifen in vitro. It is notable that NLK inhibition appears to have basal growth inhibitory activities under estrogen deprived condition in the absence of tamoxifen (Fig. 1C). This could be attributed to the repression of ER transcriptional activity following NLK inhibition under estrogen deprivation stress (Fig. 3G).
In the in vivo context, administration of VX-702 resulted in divergent responses in both the T47D-TamR xenograft tumors and the WHIM 11J PDX tumors. In both models, a majority of the tumors showed only modest responses to VX-702 mono-treatment, with the exception of a few tumors showing significant regression (Supplementary Fig. S11, 15). Protein array analysis of the residual T47D-TamR tumors following VX-702 treatment revealed active mTOR signaling in these tumors. mTOR is a key survival kinase in endocrine-resistant breast cancer, suggesting its potential role in cancer cell survival under VX-702 treatment. In light of this, we treated the T47D-TamR tumors with VX-702 in combination with everolimus, which resulted in significant tumor growth inhibition and prolonged progression free survival compared to other treatments. RPPA data of T47D-TamR tumors harvested following 2-week treatments revealed that many key signaling molecules associated with breast cancer endocrine resistance were repressed only when VX-702 was combined with everolimus. Similar significant therapeutic effects were also observed for concomitant VX-702 and everolimus treatment in the WHIM 11J ER+ PDX tumor model that harbors active NLK and mTOR signaling. These data suggest that VX-702 in combination with everolimus exhibited significant therapeutic value in acquired or de novo tamoxifen-resistant luminal breast tumor models. Of note, the preclinical models used in this study are either cell line subclone after long-term tamoxifen treatment and re-transplantation, or very aggressive cancer with a fulminant clinical course, which could limit the effectiveness of NLK inhibition.
In this present study, we have focused our mechanistic study on the modulation of genomic ER activity by NLK. Whether the high NLK-associated endocrine resistance is mediated through modulation/reprogramming of the ER transcriptional complex, and/or through modulating non-genomic ER activity, or other transcription factors and key signaling molecules, and their relative contributions to the endocrine resistance phenotype, remains to be elucidated. In addition, our in vivo studies revealed active mTOR signaling in the VX-702 resistant tumors, which provided the rationale to combine the mTOR inhibitor and VX-702. NLK is known to phosphorylate Raptor to mediate stress-induced mTORC1 inhibition (7). It is possible that NLK inhibition may activate this feedback loop in the context of in vivo tumor microenvironment, which may explain the lack of VX-702 response and the synergistic effect between Everolimus and VX-702 in xenograft tumors. Future studies will be required to elucidate the relationship between NLK and mTOR signaling in the context of endocrine resistance and under their respective targeted therapies.
Altogether, through this study, we have demonstrated the role of NLK and the therapeutic value of NLK inhibition in the context of endocrine resistance in vitro and in vivo. As new and additional genomic as well as expression data begin to emerge from endocrine-resistant metastatic tumor specimens, future studies may help understand the importance of high NLK expression/signaling in the context of acquired endocrine resistance, and assess NLK expression in paired primary and metastatic ER+ breast tumor specimens. In addition, future studies will be required to elucidate the role of NLK in aromatase inhibitor resistance and it will be interesting to examine the potential role of NLK in patients who recur with currently used CDK4/6 inhibitors. Furthermore, VX-702 is originally developed to inhibit p38 and our study revealed its off-target effect against NLK. While this drug showed strong activity against NLK in vitro, a high dosage (50 mg/kg BID) was required to achieve therapeutic effect in vivo. Future studies will be needed to test the safety profile of VX-702 at this dosage, particularly when combined with everolimus, and to develop more potent inhibitors against NLK to further improve the therapeutic effect.
Supplementary Material
Statement of Translational Significance.
Despite the tremendous success of endocrine therapies in estrogen-receptor positive breast cancer, endocrine resistance is a common and major clinical challenge. This study identified Nemo-Like Kinase (NLK), as a new actionable kinase target for endocrine-resistant breast cancer and reveals its novel function in modulating ERα transcriptional activity under endocrine stress. This study also uncovered and therapeutically evaluated a dual p38 and NLK inhibitor VX-702 in patient and cell-line derived xenograft tumor models of intrinsic and acquired endocrine resistance. This study reveals the potential benefit of targeting NLK and repurposing VX-702 for the management of the endocrine-resistant breast cancers with active NLK signaling.
Acknowledgements:
This study is supported by NIH grant 1R01CA181368 (X-S.W.), 1R01CA183976 (X-S.W.), Susan G. Komen foundation PDF15333523 (X.W.), Congressionally Directed Medical Research Program W81XWH-12-1-0166 (X-S.W.), W81XWH-12-1-0167 (R.S.), W81XWH-13-1-0201 (X-X.C), and Nancy Owens foundation. This study is also supported in part by NIH grant 1R21CA237964 (X-S.W.); CDMRP W81XWH-13-1-0431 (J.V.); Susan G. Komen foundation PDF12231561 (J.K.); the Breast Cancer Research Foundation grants BCRF-16-142, 17-143, 18-145 (R.S), Stand Up To Cancer-American Association for Cancer Research Dream Team Translational Research Grant, Grant Number SU2C-AACR-DT0409 (R.S); Commonwealth of PA Tobacco Phase 15 Formula Fund (X-S. W.), the Shear Family Foundation, and the Hillman Foundation (X-S.W); U24CA209837 (S.L.). The results published here are in part based upon data generated by The Cancer Genome Atlas project established by the NCI and NHGRI (dbGaP accession: phs000178.v6.p6). The computational infrastructure was supported by the Dan L. Duncan Comprehensive Cancer Center Biostatistics and Informatics Shared Resource, and the University of Pittsburgh Center for Research Computing. We thank the Washington University HAMLET Core for providing the tissue microarrays of WHIM PDX tumors and the WHIM11J tumor cells for re-transplantation. The RPPA experiment was supported in part by Cancer Prevention & Research Institute of Texas Proteomics & Metabolomics Core Facility Support Award (RP170005)(SH), and NCI Cancer Center Support Grant to Antibody-based Proteomics Core/Shared Resource (P30CA125123) (SH). We thank Dr. Shixia Huang, Hsin-Yi Cincy Lu, and Mr. Carlos Ramos from the Antibody-based Proteomics Core/Shared Resource for their excellent technical assistant in performing RPPA experiments. We thank Drs. Kimal Rajapakshe and Cristian Coarfa, Mr. Dimuthu Perera for RPPA data processing and normalization. We thank Dr. Bert W. O’Malley for providing recombinant SRC-3 protein.
Conflict of Interests: Dr. Rachel Schiff receives research funding from AstraZeneca, GlaxoSmithKline, Gilead Sciences, and PUMA Biotechnology, outside of this project; and is a consulting/advisory committee member for Macrogenics, and (in the past) for Eli Lilly. Dr. Shunqiang Li has received license fee from Horizon Discovery. He received research support from Pfizerr, Takeda Oncology, and Zenopharm.
Footnotes
Data Availability Statement: The TCGA data used in this study are obtained from http://cancergenome.nih.gov/ and related clinical data are obtained from https://xenabrowser.net/. The ConSig scores release 2 are available from http://consig.cagenome.org. The gene expression datasets for Loi et al., (GSE6532) (14), Symmans et al., (GSE17705) (15), and Wang et al., (GSE2034) (16) are available from http://www.ncbi.nlm.nih.gov/geo/. The RNA-seq data generated in this study is available through NCBI GEO accession number GSE141696. The codes and materials used in this study are available upon request to the corresponding author (except the materials restricted by material transfer agreements, such as the VX-702 from Vertex Pharmaceuticals Incorporated and the WHIM 11J tumors).
References
- 1.Johnston SR. New strategies in estrogen receptor-positive breast cancer. Clin Cancer Res 2010;16(7):1979–87 doi 10.1158/1078-0432.CCR-09-1823. [DOI] [PubMed] [Google Scholar]
- 2.Miller WR. Identification and mechanisms of endocrine resistance. Breast Cancer Res 2008;10 Suppl 4:S19 doi 10.1186/bcr2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annual review of medicine 2011;62:233–47 doi 10.1146/annurev-med-070909-182917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Sullivan CC. Overcoming Endocrine Resistance in Hormone-Receptor Positive Advanced Breast Cancer-The Emerging Role of CDK4/6 Inhibitors. Int J Cancer Clin Res 2015;2(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med 2011;62:233–47 doi 10.1146/annurev-med-070909-182917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishitani T, Ishitani S, Matsumoto K, Itoh M. Nemo-like kinase is involved in NGF-induced neurite outgrowth via phosphorylating MAP1B and paxillin. Journal of neurochemistry 2009;111(5):1104–18 doi 10.1111/j.1471-4159.2009.06400.x. [DOI] [PubMed] [Google Scholar]
- 7.Yuan HX, Wang Z, Yu FX, Li F, Russell RC, Jewell JL, et al. NLK phosphorylates Raptor to mediate stress-induced mTORC1 inhibition. Genes Dev 2015;29(22):2362–76 doi 10.1101/gad.265116.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang Y, Yang Y, He Y, Li J. The emerging role of Nemo-like kinase (NLK) in the regulation of cancers. Tumour Biol 2015;36(12):9147–52 doi 10.1007/s13277-015-4159-7. [DOI] [PubMed] [Google Scholar]
- 9.Emami KH, Brown LG, Pitts TE, Sun X, Vessella RL, Corey E. Nemo-like kinase induces apoptosis and inhibits androgen receptor signaling in prostate cancer cells. Prostate 2009;69(14):1481–92 doi 10.1002/pros.20998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lei L, Wang Y, Zheng YW, Fei LR, Shen HY, Li ZH, et al. Overexpression of Nemo-like kinase promotes the proliferation and invasion of lung cancer cells and indicates poor prognosis. Curr Cancer Drug Targets 2018. doi 10.2174/1568009618666181119150521. [DOI] [PubMed] [Google Scholar]
- 11.Li SZ, Zeng F, Li J, Shu QP, Zhang HH, Xu J, et al. Nemo-like kinase (NLK) primes colorectal cancer progression by releasing the E2F1 complex from HDAC1. Cancer Lett 2018;431:43–53 doi 10.1016/j.canlet.2018.05.032. [DOI] [PubMed] [Google Scholar]
- 12.Huang Y, Jiang Y, Lu W, Zhang Y. Nemo-like kinase associated with proliferation and apoptosis by c-Myb degradation in breast cancer. PloS one 2013;8(7):e69148 doi 10.1371/journal.pone.0069148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaw-Hallgren G, Chmielarska Masoumi K, Zarrizi R, Hellman U, Karlsson P, Helou K, et al. Association of nuclear-localized Nemo-like kinase with heat-shock protein 27 inhibits apoptosis in human breast cancer cells. PLoS One 2014;9(5):e96506 doi 10.1371/journal.pone.0096506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loi S, Haibe-Kains B, Desmedt C, Lallemand F, Tutt AM, Gillet C, et al. Definition of clinically distinct molecular subtypes in estrogen receptor-positive breast carcinomas through genomic grade. J Clin Oncol 2007;25(10):1239–46 doi 10.1200/JCO.2006.07.1522. [DOI] [PubMed] [Google Scholar]
- 15.Symmans WF, Hatzis C, Sotiriou C, Andre F, Peintinger F, Regitnig P, et al. Genomic index of sensitivity to endocrine therapy for breast cancer. J Clin Oncol 2010;28(27):4111–9 doi 10.1200/JCO.2010.28.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, Yang F, et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet 2005;365(9460):671–9 doi 10.1016/S0140-6736(05)17947-1. [DOI] [PubMed] [Google Scholar]
- 17.Heiser LM, Sadanandam A, Kuo WL, Benz SC, Goldstein TC, Ng S, et al. Subtype and pathway specific responses to anticancer compounds in breast cancer. Proceedings of the National Academy of Sciences of the United States of America 2012;109(8):2724–9 doi 10.1073/pnas.1018854108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrison G, Fu X, Shea M, Nanda S, Giuliano M, Wang T, et al. Therapeutic potential of the dual EGFR/HER2 inhibitor AZD8931 in circumventing endocrine resistance. Breast Cancer Res Treat 2014;144(2):263–72 doi 10.1007/s10549-014-2878-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu X, Jeselsohn R, Pereira R, Hollingsworth EF, Creighton CJ, Li F, et al. FOXA1 overexpression mediates endocrine resistance by altering the ER transcriptome and IL-8 expression in ER-positive breast cancer. Proc Natl Acad Sci U S A 2016;113(43):E6600–E9 doi 10.1073/pnas.1612835113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nardone A, Weir H, Delpuech O, Brown H, De Angelis C, Cataldo ML, et al. The oral selective oestrogen receptor degrader (SERD) AZD9496 is comparable to fulvestrant in antagonising ER and circumventing endocrine resistance. Br J Cancer 2019;120(3):331–9 doi 10.1038/s41416-018-0354-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 2003;30(3):256–68. [DOI] [PubMed] [Google Scholar]
- 22.Kim JA, Tan Y, Wang X, Cao X, Veeraraghavan J, Liang Y, et al. Comprehensive functional analysis of the tousled-like kinase 2 frequently amplified in aggressive luminal breast cancers. Nat Commun 2016;7:12991 doi 10.1038/ncomms12991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trista Schagat APaKK. Normalizing Genetic Reporter Assays Approaches and Considerations for Increasing Consistency and Statistical Significance. Cell Notes 2007:9–12. [Google Scholar]
- 24.Wu RC, Feng Q, Lonard DM, O’Malley BW. SRC-3 coactivator functional lifetime is regulated by a phospho-dependent ubiquitin time clock. Cell 2007;129(6):1125–40 doi 10.1016/j.cell.2007.04.039. [DOI] [PubMed] [Google Scholar]
- 25.Basu D, Bewley AF, Sperry SM, Montone KT, Gimotty PA, Rasanen K, et al. EGFR inhibition promotes an aggressive invasion pattern mediated by mesenchymal-like tumor cells within squamous cell carcinomas. Mol Cancer Ther 2013;12(10):2176–86 doi 10.1158/1535-7163.MCT-12-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hai J, Zhu CQ, Wang T, Organ SL, Shepherd FA, Tsao MS. TRIM14 is a Putative Tumor Suppressor and Regulator of Innate Immune Response in Non-Small Cell Lung Cancer. Sci Rep 2017;7:39692 doi 10.1038/srep39692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schober P, Vetter TR. Repeated Measures Designs and Analysis of Longitudinal Data: If at First You Do Not Succeed-Try, Try Again. Anesth Analg 2018;127(2):569–75 doi 10.1213/ANE.0000000000003511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Comprehensive molecular portraits of human breast tumours. Nature 2012;490(7418):61–70 doi 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chi X, Sartor MA, Lee S, Anurag M, Patil S, Hall P, et al. Universal concept signature analysis: genome-wide quantification of new biological and pathological functions of genes and pathways. Brief Bioinform 2019. doi 10.1093/bib/bbz093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang XS, Prensner JR, Chen G, Cao Q, Han B, Dhanasekaran SM, et al. An integrative approach to reveal driver gene fusions from paired-end sequencing data in cancer. Nat Biotechnol 2009;27(11):1005–11 doi 10.1038/nbt.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sotiriou C, Neo SY, McShane LM, Korn EL, Long PM, Jazaeri A, et al. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci U S A 2003;100(18):10393–8 doi 10.1073/pnas.1732912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gill S, Sargent D. End points for adjuvant therapy trials: has the time come to accept disease-free survival as a surrogate end point for overall survival? Oncologist 2006;11(6):624–9 doi 10.1634/theoncologist.11-6-624. [DOI] [PubMed] [Google Scholar]
- 33.THERNEAU TM, GRAMBSCH PM, FLEMING TR. Martingale-based residuals for survival models Biometrika 1990;77(1):147–60. [Google Scholar]
- 34.Pei-Yun Chuang CH, Huang Hsiu-Chen. The use of a combination of tamoxifen and doxorubicin synergistically to induce cell cycle arrest in BT483 cells by down-regulating CDK1, CDK2 and cyclin D expression. Journal of Pharmaceutical Technology & Drug Research 2013;2:7 doi doi: 10.7243/2050-120X-2-12. [DOI] [Google Scholar]
- 35.Reddel RR, Murphy LC, Hall RE, Sutherland RL. Differential sensitivity of human breast cancer cell lines to the growth-inhibitory effects of tamoxifen. Cancer Res 1985;45(4):1525–31. [PubMed] [Google Scholar]
- 36.Ji H, Stout LE, Zhang Q, Zhang R, Leung HT, Leung BS. Absence of transforming growth factor-beta responsiveness in the tamoxifen growth-inhibited human breast cancer cell line CAMA-1. J Cell Biochem 1994;54(3):332–42 doi 10.1002/jcb.240540309. [DOI] [PubMed] [Google Scholar]
- 37.Wishart DS, Knox C, Guo AC, Shrivastava S, Hassanali M, Stothard P, et al. DrugBank: a comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res 2006;34(Database issue):D668–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen X, Ji ZL, Chen YZ. TTD: Therapeutic Target Database. Nucleic Acids Res 2002;30(1):412–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science 2002;298(5600):1912–34 doi 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 40.Fleischmann A, Darsow M, Degtyarenko K, Fleischmann W, Boyce S, Axelsen KB, et al. IntEnz, the integrated relational enzyme database. Nucleic Acids Res 2004;32(Database issue):D434–7 doi 10.1093/nar/gkh11932/suppl_1/D434 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anastassiadis T, Deacon SW, Devarajan K, Ma H, Peterson JR. Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity. Nature biotechnology 2011;29(11):1039–45 doi 10.1038/nbt.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Damjanov N, Kauffman RS, Spencer-Green GT. Efficacy, pharmacodynamics, and safety of VX-702, a novel p38 MAPK inhibitor, in rheumatoid arthritis: results of two randomized, double-blind, placebo-controlled clinical studies. Arthritis Rheum 2009;60(5):1232–41 doi 10.1002/art.24485. [DOI] [PubMed] [Google Scholar]
- 43.Anastassiadis T, Deacon SW, Devarajan K, Ma H, Peterson JR. Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity. Nat Biotechnol 2011;29(11):1039–45 doi 10.1038/nbt.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ishitani S, Inaba K, Matsumoto K, Ishitani T. Homodimerization of Nemo-like kinase is essential for activation and nuclear localization. Molecular biology of the cell 2011;22(2):266–77 doi 10.1091/mbc.E10-07-0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen L, Mayer JA, Krisko TI, Speers CW, Wang T, Hilsenbeck SG, et al. Inhibition of the p38 kinase suppresses the proliferation of human ER-negative breast cancer cells. Cancer Res 2009;69(23):8853–61 doi 10.1158/0008-5472.CAN-09-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gutierrez MC, Detre S, Johnston S, Mohsin SK, Shou J, Allred DC, et al. Molecular changes in tamoxifen-resistant breast cancer: relationship between estrogen receptor, HER-2, and p38 mitogen-activated protein kinase. J Clin Oncol 2005;23(11):2469–76 doi 10.1200/JCO.2005.01.172. [DOI] [PubMed] [Google Scholar]
- 47.Garee JP, Meyer R, Oesterreich S. Co-repressor activity of scaffold attachment factor B1 requires sumoylation. Biochemical and biophysical research communications 2011;408(4):516–22 doi 10.1016/j.bbrc.2011.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mak P, Leung YK, Tang WY, Harwood C, Ho SM. Apigenin suppresses cancer cell growth through ERbeta. Neoplasia 2006;8(11):896–904 doi 10.1593/neo.06538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maruyama K, Endoh H, Sasaki-Iwaoka H, Kanou H, Shimaya E, Hashimoto S, et al. A novel isoform of rat estrogen receptor beta with 18 amino acid insertion in the ligand binding domain as a putative dominant negative regular of estrogen action. Biochemical and biophysical research communications 1998;246(1):142–7 doi 10.1006/bbrc.1998.8590. [DOI] [PubMed] [Google Scholar]
- 50.de Leeuw R, Neefjes J, Michalides R. A role for estrogen receptor phosphorylation in the resistance to tamoxifen. Int J Breast Cancer 2011;2011:232435 doi 10.4061/2011/232435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amazit L, Pasini L, Szafran AT, Berno V, Wu RC, Mielke M, et al. Regulation of SRC-3 intercompartmental dynamics by estrogen receptor and phosphorylation. Molecular and cellular biology 2007;27(19):6913–32 doi 10.1128/MCB.01695-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gianni M, Parrella E, Raska I Jr., Gaillard E, Nigro EA, Gaudon C, et al. P38MAPK-dependent phosphorylation and degradation of SRC-3/AIB1 and RARalpha-mediated transcription. The EMBO journal 2006;25(4):739–51 doi 10.1038/sj.emboj.7600981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu RC, Qin J, Yi P, Wong J, Tsai SY, Tsai MJ, et al. Selective phosphorylations of the SRC-3/AIB1 coactivator integrate genomic reponses to multiple cellular signaling pathways. Molecular cell 2004;15(6):937–49 doi 10.1016/j.molcel.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 54.Ding C. Drug evaluation: VX-702, a MAP kinase inhibitor for rheumatoid arthritis and acute coronary syndrome. Curr Opin Investig Drugs 2006;7(11):1020–5. [PubMed] [Google Scholar]
- 55.Morrison G, Fu X, Shea M, Nanda S, Giuliano M, Wang T, et al. Therapeutic potential of the dual EGFR/HER2 inhibitor AZD8931 in circumventing endocrine resistance. Breast cancer research and treatment 2014;144(2):263–72 doi 10.1007/s10549-014-2878-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Emami KH, Brown LG, Pitts TE, Sun X, Vessella RL, Corey E. Nemo-like kinase induces apoptosis and inhibits androgen receptor signaling in prostate cancer cells. The Prostate 2009;69(14):1481–92 doi 10.1002/pros.20998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barnett SW, Barley J, Francis R, Lane S, Gardner RL, John W, et al. Manual of animal technology. Oxford: Blackwell Publishing; 2008. [Google Scholar]
- 58.Osborne CK, Wakeling A, Nicholson RI. Fulvestrant: an oestrogen receptor antagonist with a novel mechanism of action. Br J Cancer 2004;90 Suppl 1:S2–6 doi 10.1038/sj.bjc.6601629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ciruelos E, Pascual T, Arroyo Vozmediano ML, Blanco M, Manso L, Parrilla L, et al. The therapeutic role of fulvestrant in the management of patients with hormone receptor-positive breast cancer. Breast 2014;23(3):201–8 doi 10.1016/j.breast.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 60.Sekulic A, Hudson CC, Homme JL, Yin P, Otterness DM, Karnitz LM, et al. A direct linkage between the phosphoinositide 3-kinase-AKT signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res 2000;60(13):3504–13. [PubMed] [Google Scholar]
- 61.Baselga J, Semiglazov V, van Dam P, Manikhas A, Bellet M, Mayordomo J, et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol 2009;27(16):2630–7 doi 10.1200/JCO.2008.18.8391. [DOI] [PubMed] [Google Scholar]
- 62.Li S, Shen D, Shao J, Crowder R, Liu W, Prat A, et al. Endocrine-therapy-resistant ESR1 variants revealed by genomic characterization of breast-cancer-derived xenografts. Cell reports 2013;4(6):1116–30 doi 10.1016/j.celrep.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chow A, Arteaga CL, Wang SE. When tumor suppressor TGFbeta meets the HER2 (ERBB2) oncogene. Journal of mammary gland biology and neoplasia 2011;16(2):81–8 doi 10.1007/s10911-011-9206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fu CH, Lin RJ, Yu J, Chang WW, Liao GS, Chang WY, et al. A novel oncogenic role of inositol phosphatase SHIP2 in ER-Negative breast cancer stem cells: Involvement of JNK/Vimentin activation. Stem cells 2014. doi 10.1002/stem.1735. [DOI] [PubMed] [Google Scholar]
- 65.Schiff R, Reddy P, Ahotupa M, Coronado-Heinsohn E, Grim M, Hilsenbeck SG, et al. Oxidative stress and AP-1 activity in tamoxifen-resistant breast tumors in vivo. J Natl Cancer Inst 2000;92(23):1926–34. [DOI] [PubMed] [Google Scholar]
- 66.Malorni L, Giuliano M, Migliaccio I, Wang T, Creighton CJ, Lupien M, et al. Blockade of AP-1 Potentiates Endocrine Therapy and Overcomes Resistance. Mol Cancer Res 2016;14(5):470–81 doi 10.1158/1541-7786.MCR-15-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science 1995;270(5241):1491–4. [DOI] [PubMed] [Google Scholar]
- 68.Osborne CK, Schiff R, Fuqua SA, Shou J. Estrogen receptor: current understanding of its activation and modulation. Clin Cancer Res 2001;7(12 Suppl):4338s–42s; discussion 411s-412s. [PubMed] [Google Scholar]
- 69.Garcia-Becerra R, Santos N, Diaz L, Camacho J. Mechanisms of resistance to endocrine therapy in breast cancer: focus on signaling pathways, miRNAs and genetically based resistance. Int J Mol Sci 2012;14(1):108–45 doi 10.3390/ijms14010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Han H, Cho JW, Lee S, Yun A, Kim H, Bae D, et al. TRRUST v2: an expanded reference database of human and mouse transcriptional regulatory interactions. Nucleic Acids Res 2018;46(D1):D380–D6 doi 10.1093/nar/gkx1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lin CY, Strom A, Vega VB, Kong SL, Yeo AL, Thomsen JS, et al. Discovery of estrogen receptor alpha target genes and response elements in breast tumor cells. Genome Biol 2004;5(9):R66 doi 10.1186/gb-2004-5-9-r66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.