Figure 7. IFN-γ produced by macrophages and NK cells is required for the therapeutic efficacy of 1X AIP.
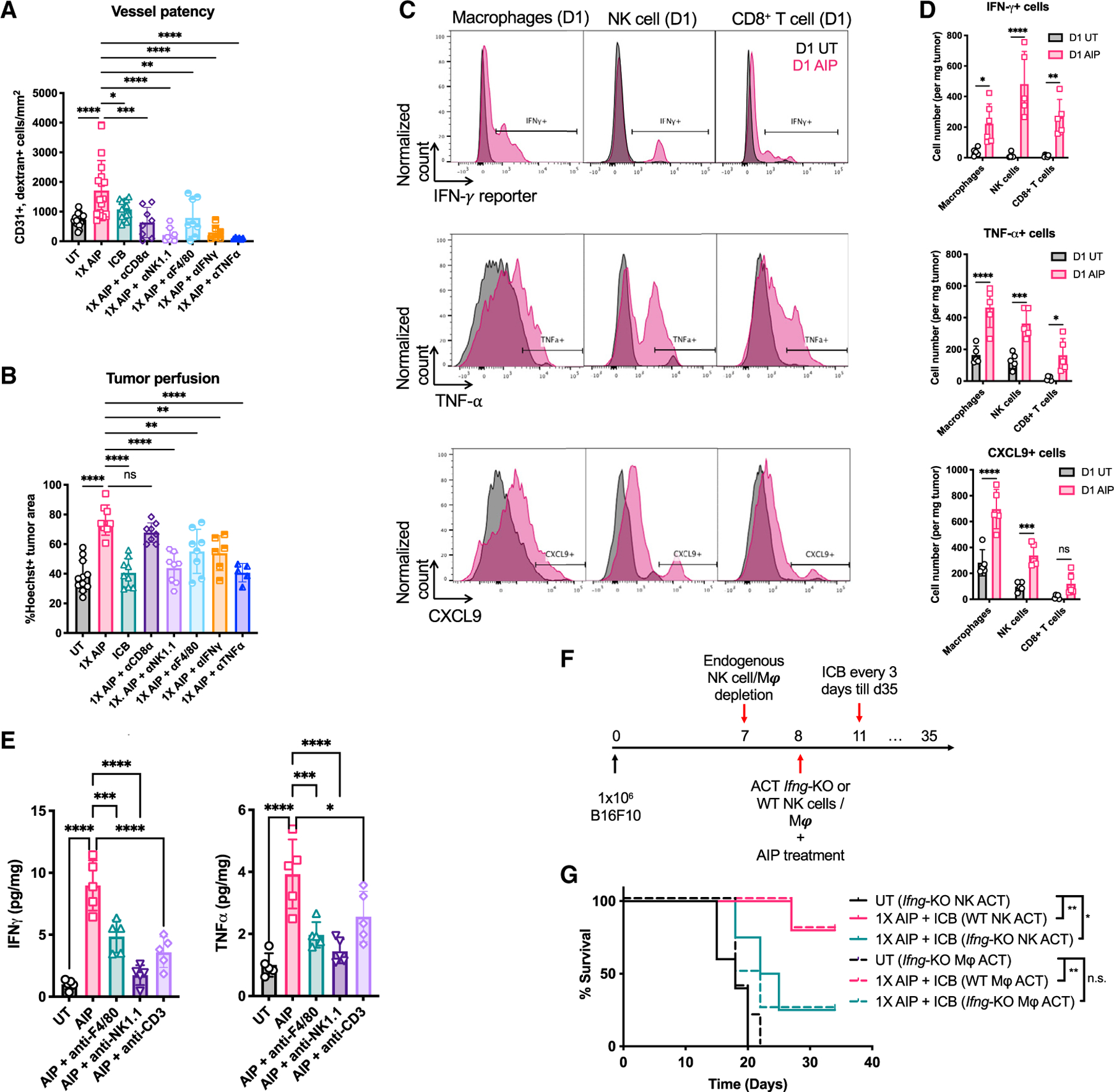
(A–E) Mice bearing B16F10 tumors (n = 5–6 animals/group, pooled from at least two independent experiments) were left UT, treated with ICB alone, or treated with 1X AIP + ICB as in Figure 1G. Some groups received depleting antibodies against NK cells, macrophages, or IFN-γ, TNF-⍺ beginning 1 day prior to treatment as indicated.
(A and B) Shown are quantifications of vessel patency (CD31+dextran+ cells/mm2; A) and tumor perfusion (Hoechst+ % area; B) levels in tumor sections.
(C and D) B16F10-bearing IFN-γ reporter mice were UT or treated with 1X AIP as in Figure 1G. Tumors were collected 1 day post treatment. The numbers of IFN-γ-, TNF-⍺- and CXCL9-secreting macrophages, NK cells, and CD8+ T cells were determined by flow cytometry.
(E) Quantification of IFN-γ and TNF-⍺ levels in B16F10-bearing mice 1-day post AIP treatment in the presence of indicated depleting antibodies.
(F and G) Mice bearing B16F10 tumors (n = 5 animals/group) were depleted of endogenous NK cells or macrophages one day prior to treatment with 1X AIP + ICB. On the day of treatment, animals received adoptive transfer of WT or IFN-γ-deficient macrophages (3–4 × 106) or NK cells (1 × 106). Shown is the experiment timeline (F) and survival of these mice over time (G).
Throughout, *p < 0.05; **p < 0.01; **p < 0.001; ****p < 0.0001 determined by one-way ANOVA with Dunnett post test (A, B, and E), two-way ANOVA followed by Holm-Šídák test (D), and log rank test (G). All error bars are SD. See also Figure S7.
