Abstract
Low-quality dietary patterns impair cardiometabolic health by increasing the risk of obesity-related disorders. Cardiometabolic risk relative to dairy-food consumption continues to be a controversial topic, due to recommendations that endorse low-fat and nonfat dairy foods over full-fat varieties despite accumulated evidence that does not strongly support these recommendations. Controlled human studies and mechanistic preclinical investigations support that full-fat dairy foods decrease cardiometabolic risk by promoting gut health, reducing inflammation, and managing dyslipidemia. These gut- and systemic-level cardiometabolic benefits are attributed, at least in part, to milk polar lipids (MPLs) derived from the phospholipid- and sphingolipid-rich milk fat globule membrane that is of higher abundance in full-fat dairy milk. The controversy surrounding full-fat dairy food consumption is discussed in this review relative to cardiometabolic health and MPL bioactivities that alleviate dyslipidemia, shift gut microbiota composition, and reduce inflammation. This summary, therefore, is expected to advance the understanding of full-fat dairy foods through their MPLs and the need for translational research to establish evidence-based dietary recommendations.
Keywords: dairy milk, milk fat globule membrane, milk phospholipids, milk polar lipids, milk sphingomyelin
INTRODUCTION
A substantial proportion of the global population has poor cardiometabolic health. This is reflected by the high prevalence rates of numerous, interrelated cardiometabolic diseases and disorders (eg, obesity, prediabetes and diabetes, metabolic syndrome [MetS], and nonalcoholic fatty liver disease [NAFLD]).1–3 Costs to manage these conditions also pose a substantial societal burden, with expenditures in the United States alone exceeding trillions of dollars annually for the direct and indirect costs of obesity, type 2 diabetes mellitus (T2DM), and cardiovascular disease (CVD).4 Critical to improving human health is establishing effective dietary practices that can alleviate the risk of developing these disorders or that can reverse their progression toward causing premature death. This is especially important because many persons with impaired cardiometabolic health have subclinical conditions (eg, prediabetes, MetS), which are often left unmanaged other than to encourage lifestyle modification. Although numerous foods are rich in bioactive components and have the potential to improve cardiometabolic health, bovine-derived dairy foods have been touted for their low cost and nutrient-rich matrix of high-quality proteins and micronutrients.5
Although humans are the only mammal who drink dairy milk throughout their lifespan, its consumption is known to decrease in an age-dependent manner.6 Furthermore, recommendations support 3 daily servings of dairy foods to help achieve nutritional adequacy of several essential vitamins and minerals, but American adults are estimated to only achieve ∼50% of this amount.7 Indeed, estimates indicate that if Americans were to meet dairy recommendations, billions of dollars could be saved annually from a reduction in several cardiometabolic diseases and certain cancers and neurological disorders.7 Despite the potential of dairy foods to thwart cardiometabolic risks, controversy surrounds recommendations that encourage low-fat or nonfat varieties over full-fat dairy foods to reduce the intake of saturated fat and maintain energy balance.8 This controversy exists because compelling evidence supports full-fat dairy foods to protect against cardiometabolic disorders (eg, obesity, MetS, T2DM), whereas outcomes of relatively few studies are neutral or suggest adverse effects.9
The specific components of full-fat dairy foods responsible for their putative health benefits are not well established, but the milk fat globule membrane (MFGM) has received significant attention.10 The MFGM is a unique, trilayer membrane that contains the milk polar lipids (MPLs; ie, milk phospholipids and sphingolipids) and surrounds the lipids of the milk fat globule (MFG) core. MPLs are abundant in certain higher-fat dairy foods but can be reduced during the commercial processing that produces lower-fat dairy foods.10 The reduced dietary availability of MPLs raises health concerns because they show promise to favorably influence intraluminal lipid emulsions,11 gut barrier functions,12 dyslipidemia,13 and inflammation14 toward improved cardiometabolic health. Therefore, in this review, we discuss the controversy surrounding full-fat bovine dairy-food consumption relative to cardiometabolic risk and the reported bioactivities of MPLs that protect against dyslipidemia, improve gut microbiota composition, and decrease gut and systemic inflammation. This summary should advance the understanding of the health benefits of full-fat bovine dairy foods mediated through their MPLs and the need for translational research to establish evidence-based dietary recommendations.
CARDIOMETABOLIC HEALTH
Cardiometabolic health is the term that describes the metabolic and cardiovascular health status of an individual.15 Metabolic diseases are often characterized by mitochondrial dysfunction and altered host metabolism, which are implicated in insulin resistance, T2DM, NAFLD, obesity, and MetS. Whereas, CVD, including hypertension, cerebrovascular disease, and coronary artery disease, describes disorders of the heart and circulation.16 Importantly, cardiometabolic disorders precede overt CVD, consistent with evidence that persons with MetS are twice as likely to develop CVD than those without MetS.17,18
The high prevalence of cardiometabolic disorders is of substantial concern. Indeed, as is the case globally,19 CVD is leading cause of death in the United States, with an estimated 121.5 million adults having some form of this disease.20 The prevalence of obesity has increased by 20% from 1988 to 2018 and now affects 42.4% of Americans.21 MetS afflicts ∼35% of all adults, and disproportionately affects those older than 60 y (48.6%) compared with 19.5% of those aged 20–39 y.22 Consistent with NAFLD being the hepatic manifestation of MetS, an estimated 80 million to 100 million cases exist23 and 10.8 million new cases are predicted by 2060, along with an economic burden of $350 billion.24 Given the close association between MetS and diabetes, it is not surprising that age-related trends for T2DM are similar, with 4.2% of the 34.2 million diagnosed cases being among young adults (aged 18–44 y) vs 26.8% among those > 65 y of age.25 Also, 34.5% of US adults have prediabetes,25 suggesting that the incidence of T2DM will increase in the coming years. Although some overlap in the prevalence of these cardiometabolic disorders exists, it is unquestionable that a substantial proportion of the population has compromised cardiometabolic health, and this drives efforts to mitigate the risk for premature death.
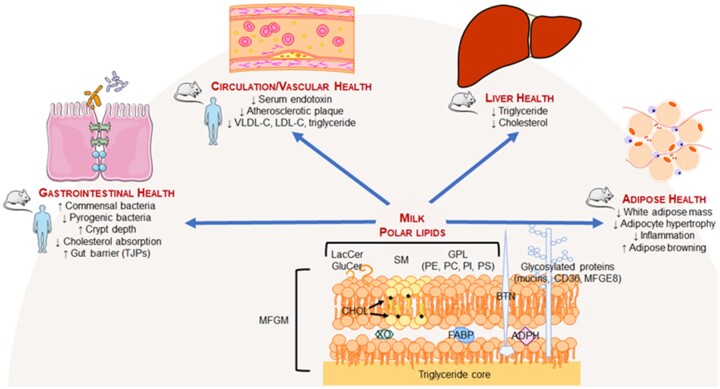
Although CVD-related death has a high incidence, 90% of its known risk factors (eg, dyslipidemia, hypertension, obesity, insulin resistance) are modifiable by diet and lifestyle.26 A common underlying etiology of insulin resistance and impaired cardiometabolic health is chronic, low-grade inflammation,27 including that attributed to postprandial metabolism. For example, during a meal, the intestinal lumen is exposed to complex foodstuffs (eg, macronutrients, saturated fatty acids), gut hormones, and bacteria. Low-quality dietary patterns (eg, Western diet,28 typical American diet29) are implicated in hyperglycemia and hyperlipidemia.30 Chronic excursions in blood glucose provoke the formation of advanced glycation end-products that induce damage to the vascular endothelium and exacerbate insulin resistance.31 In close association with hyperglycemia and hyperlipidemia is metabolic endotoxemia, which results from the translocation of gut-derived endotoxins (eg, lipopolysaccharides derived from gram-negative bacteria) to the systemic circulation where it can bind cellular Toll-like receptor-4 to initiate NF-κB–dependent inflammation32 and provoke cardiometabolic risk. That poor quality diets drive chronic inflammation supports the concept that dietary modification could have substantial influence on mitigating cardiometabolic risks. Although numerous dietary factors can positively influence this paradigm, we describe in this review benefits of bovine dairy foods, with a focus on their controversial aspects: fat-free and nonfat varieties of dairy are recommended over full-fat varieties for a healthy dietary pattern6,33 despite evidence that full-fat dairy may reduce cardiometabolic risks.34 Importantly, we highlight in this review the bioactivities of MPLs (Figure 1) found in the MFGM that surrounds the triglyceride-rich MFG.
Figure 1.
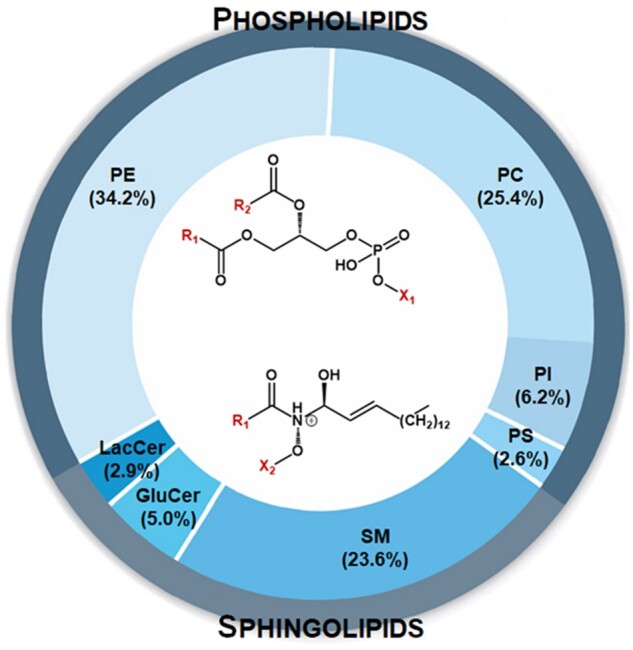
Distribution of polar lipids in raw milk from cows. The polar lipid content of bovine milk is 12.8–40.0 mg/100 g, with total phospholipid concentrations approximately doubling those of total sphingolipid concentrations.35,36 Phospholipids contain a phosphoglycerol backbone, with a saturated fatty acid at R1, an unsaturated fatty acid at R2, and 1 of several alcohols (ie, ethanolamine, serine, inositol, or choline) at the polar head of X1. Sphingolipids consist of an amide-containing sphingoid backbone (primarily sphingosine in milk). Similar to phospholipids, R1 is a saturated fatty acid, whereas X2 is substituted with phosphocholine, phosphoethanolamine, or a sugar. Abbreviations: GluCer, glucosyl ceramide; LacCer, lactosyl ceramide; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; PS, phosphatidylserine; SM, sphingomyelin
BOVINE DAIRY-MILK COMPOSITION
Bovine dairy milk is well recognized for its nutrient-rich matrix of macronutrients and essential micronutrients.33 The principal components of dairy milk, including its water, fat, proteins, and carbohydrate, were characterized during the early 20th century.37 Its proteins are primarily casein (80%) and whey protein (20%), and its carbohydrate mainly is lactose.37 Milk proteins contain all 9 essential amino acids to make it a high-quality protein source.33 Proteomic analysis has also revealed hundreds of milk proteins, which have been reviewed elsewhere.38–40 Separate from the major proteins of casein and whey, bovine milk contains membrane proteins in the MFGM. These proteins constitute 1%–4% of the total protein content and exist in a 3:2 ratio with MFGM polar lipids.41 These minor proteins include lactadherin (or MFG–epidermal growth factor-8 [EGF-8]) > xanthine oxidase > butyrophilin, and varying quantities of mucin 1, cluster of differentiation 36, adipophilin, fatty acid–binding protein, and protease peptone 3, which have been reviewed elsewhere.42,43 Milk fat exists primarily as triglycerides (∼98%); the remaining 2% presents as diacylglycerol, phospholipids (∼1%), cholesterol, and free fatty acids.44 Milk fatty acids are predominantly saturated fatty acids (70%), followed by monounsaturated fatty acids (25%) and polyunsaturated fatty acids (3.4%).45 Dairy milk also contains macrominerals, trace elements, and water- and fat-soluble vitamins. Importantly, the nutrient-dense matrix of dairy milk can help achieve nutritional adequacy for several nutrients (namely, calcium, magnesium, phosphorous, vitamin A, vitamin B12, zinc, potassium, and choline).46
The lipid profile of raw milk is variable, due to its dependence on the diet and the microbial populations in the rumen of the cow. The application of biochemical techniques in the early 1900s simplified the approach to assess the fat content of dairy milk, which enabled an understanding that its fat content varied between 2% and 5%.47 Modern-day chromatographic and mass spectrometry techniques have permitted the identification of > 400 types of fatty acids from dairy milk, with only 12 fatty acids present at proportions > 1%.48 Palmitic acid (C16:0; ie, the carbon chain of palmitic acid consists of 16 carbon atoms and there are no double bonds), the most abundant saturated fatty acid, contributes 28%–34% of the total fatty acid species. Thereafter, myristic acid (C14:0; 11%) and stearic acid (C18:0; 12%) are the next most abundant fatty acids.48,49 Of the unsaturated fatty acids, oleic acid (C18:1) predominates, at 20%–25% of all fatty acids.44,48
The MFGM contains 2 major classes of polar lipids that account for ∼1% of total lipids in milk (Figure 1). Milk phospholipids primarily contain a glycerol backbone as glycerophospholipids, whereas sphingolipids have a sphingoid base (eg, sphingosine).35 Glycerophospholipids differ in their polar head groups, and, in order, the most abundant in dairy milk are phosphatidylethanolamine > phosphatidylcholine > phosphatidylinositol > phosphatidylserine. Sphingolipids also possess unique polar head groups. For example, sphingomyelin (SM), which is both a phospholipid and sphingolipid (ie, a phosphosphingolipid) and represents ∼25% of total MPLs,10 can be formed by a phosphodiester bond with either phosphocholine or phosphoethanolamine. Glycolipids are another subclass of sphingolipids that are formed by glycosidic linkage of glucose at the C1 hydroxyl position of a sphingoid base to yield glucosylceramide; lactosylceramide is generated by the addition of a galactose residue. Glucosylceramide and lactosylceramide are also known as cerebrosides.50 Phospholipids differ structurally from sphingolipids. They contain 2 esterified fatty acids, whereas sphingolipids are characterized by a single amide-linked fatty acid on the sphingoid base backbone.36 The fatty acid substitutions on phospholipids are variable, but the sn-1 position tends to be a saturated fatty acid, whereas an unsaturated fatty is often at the sn-2 position.51 Sphingolipids, with their single fatty acid, tend to contain saturated fatty acids. Morrison et al52 reported that the predominant N-linked fatty acids on sphingosine are C18:0, C22:0, and C24:0, which is in alignment with recent reports that long-chain and very-long-chain saturated fatty acids (C16:0 to C24:0) are common in sphingolipids.53 The fatty acid profile of milk triglyceride also differs from that of MPLs in that short-chain fatty acids (C4:0, C6:0, C8:0) are virtually absent in MPLs, whereas C16:0, C18:0, C18:1, and C18:2 are common.53,54
It is important to note that commercial processing substantially affects MFGM and its membrane-associated polar lipids. Fresh whole milk (3.5% fat with 35 mg of MFGM per 100 g) is used to produce cream (38% fat with 200 mg of MFGM per 100 g) by separating the milk-fat layer from milk before homogenization.10 Cream can be churned into butter, but this mechanically vigorous process results in a butter product that has very little polar lipid content.55 Buttermilk, the co-product of churning cream, has high MPL levels compared with other dairy products.55 Indeed, buttermilk contains 2.03 g MPL per 100 g dry matter compared with 0.17–0.26 g in butter.10 That buttermilk is richer in MPLs than butter also explains why MFGM is often isolated from buttermilk for commercial use. Not only is the MFGM ingredient used to manufacture MFGM-enriched dairy products, it is also used in infant formula in an attempt to recapitulate the polar lipid content of breast milk.56 Importantly, because the MFGM surrounds fat globules of milk, it is not surprising that the removal of dairy fat to manufacture low-fat and nonfat milk significantly reduces total MPL levels by up to 40% (Figure 2). Although the intent is to reduce dietary energy and saturated fat content, this practice of defatting milk may also contribute to the controversy concerning dairy foods and cardiometabolic health, because it also depletes health-promoting MPLs.
Figure 2.
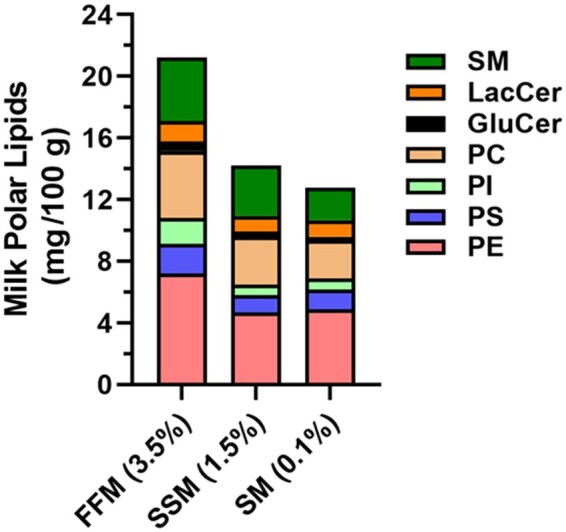
Milk polar lipid content in fluid cow’s milk. Although the relative proportions of specific milk polar lipids are unaffected, the total quantity of milk polar lipid is reduced by ≤40% in SSM (1.5% fat, weight per weight) and skim milk (0.1%) compared with FFM (3.5%). Data from Rombaut et al.55Abbreviations: FFM, full-fat milk; GluCer, glucosyl ceramide; LacCer, lactosyl ceramide; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; PS, phosphatidylserine; SM, sphingomyelin; SSM, semi-skim milk
CONTROVERSY SURROUNDING THE CONSUMPTION OF DAIRY AND DAIRY FAT ON CARDIOMETABOLIC HEALTH
The risk -benefit relationship between cardiometabolic disorders and dairy foods has received significant attention. This paradigm has been assessed in observational studies, both retrospective and prospective, and evidence continues to accumulate from the relatively fewer controlled trials in humans. The work in this area has considered the potential benefits of whole dairy foods, including lower-fat and higher-fat varieties, as well as the macronutrient, micronutrient, and other bioactive components in whole dairy foods that are thought to influence cardiometabolic health. However, in this section, we focus on the ongoing scientific controversy of whether the consumption of full-fat dairy foods adversely affects cardiometabolic health.
In support of the benefits of dairy foods, but without consideration of fat content, results of a meta-analysis indicated that higher dairy intake among cohort studies (relative risk [RR] = 0.86; 95%CI: 0.79–0.92) and cross-sectional and case-control studies (odds ratio [OR] = 0.83; 95%CI: 0.73–0.94) was associated with a lower risk of MetS.57 Dose-response analysis of prospective studies also suggested a 6% lower risk of MetS with each 1-serving increment of dairy consumption (RR = 0.94; 95%CI: 0.90–0.98).57 Another meta-analysis of cohort studies58 that expanded upon earlier meta-analyses conducted by the same authors59–61 confirmed that higher intake of total dairy (RR = 0.97; 95%CI: 0.95–1.00) and low-fat dairy (RR = 0.96; 95%CI: 0.92–1.00) corresponded to a 3%–4% lower risk of T2DM. Dairy food–specific associations were detected such that yogurt consumption was associated with a lower T2DM risk (RR = 0.86; 95%CI: 0.83–0.90; P < 0.001) and each 200 g intake of dairy milk was associated with an 8% lower risk of stroke (RR = 0.92; 95%CI: 0.88–0.97).58 In a large, multicountry cohort study of > 136 000 individuals, greater intake of dairy food (> 2 servings/d and regardless of fat content) compared with diets devoid of any dairy foods were associated with a lower risk of cardiovascular-associated death (RR = 0.77; 95%CI: 0.58–1.01; P = 0.029), major cardiovascular events (hazard ratio [HR] = 0.78; 95%CI: 0.67–0.90; P = 0.0001), and stroke (HR = 0.66; 95%CI: 0.53–0.82; P = 0.0003); these relations were observed without any altered risk of myocardial infarction.62 In this study, associations were also influenced by intake of specific dairy foods. Greater milk (HR = 0.90; 95%CI: 0.82–0.99; P = 0.053) and yogurt consumption (for > 1 vs 0 servings/d, HR = 0.86; 95%CI: 0.75–0.99; P = 0.0051) corresponded with a lower risk of several cardiovascular-related end points, whereas neither cheese nor butter consumption correlated with these cardiovascular-related outcomes. Similarly, evidence from a cross-sectional study of Portuguese adolescents (aged 15–18 y; n = 494) showed that those with higher dairy-milk intake had significantly lower cardiometabolic risk scores (based on summing z-scores of all individual risk factors) than those with lower dairy-milk intake (10.6% vs 18.1%).63 Likewise, those with appropriate dairy intake (defined as being above the median for the population) were less likely to have elevated cardiometabolic risk scores (OR = 0.53; 95%CI: 0.30–0.93; P < 0.027). However, cardiometabolic risk scores of these persons were not associated with intake of total dairy, yogurt, or cheese. This finding suggests that the observed health benefits are milk specific and potentially reflect complex interactions in the overall dietary pattern.
Not all observational studies have detected positive outcomes for dairy-food consumption. In a prospective study of biracial urban adults (aged 30–64 y; n = 1371), researchers examined baseline and the annual change in dairy intake relative to cardiometabolic risk.64 Cheese (HR = 1.13; 95%CI: 1.05–1.23; P < 0.05) and yogurt (HR = 1.21; 95%CI: 1.01–1.44; P < 0.05) consumption at baseline and the annual change in cheese (HR = 1.90; 95%CI: 1.47–2.47; P < 0.05) and yogurt (HR = 1.21; 95%CI: 1.01–1.44; P < 0.05) consumption were associated with an increased risk of central obesity. Central obesity risk, however, was unrelated to the consumption of total milk at baseline or its annual change. Baseline fluid-milk consumption, however, was associated with lower risk of MetS (HR = 0.86; 95%CI: 0.78–0.94; P < 0.05), with favorable associations specifically observed for high-density lipoprotein cholesterol–related dyslipidemia (HR = 1.10; 95%CI: 1.01–1.21; P < 0.05). In contrast, the annual change in dairy-fat consumption from all foods (based on myristic acid [C14:0] intake relative to total fat consumption) was positively associated with MetS, specifically triglyceride and high-density lipoprotein cholesterol dyslipidemias, but not with other MetS criteria (ie, central obesity, hypertension, hyperglycemia).64 Despite these disparate observations by dairy-food type, a strength of this study was its prospective assessment of dietary intake on 2 separate occasions, which would be expected to reduce measurement error of dietary variables. However, the authors acknowledged that dairy-food intake by the low-socioeconomic-status cohort in their study was approximately half a serving les than the national average. Furthermore, only 5% of these persons achieved the recommended 3 daily servings of dairy consumption, which may have influenced the observed relations.
Other cohort studies have also assessed the relationship of MetS risk with dairy-food intake but have considered whether full-fat dairy foods influence this association. The PURE study was a large-scale, multinational, prospective cohort study that examined the dietary habits of adults (aged 35–70 y) over a median follow-up of 9.1 years.65 Findings suggested that ≥2 servings/d of dairy foods compared with zero daily servings corresponded to a 24% lower MetS risk (OR = 0.76; 95%CI: 0.71–0.80; P < 0.0001). Higher intake of whole-fat dairy alone (OR = 0.72; 95%CI: 0.66–0.78; P < 0.0001) or when consumed as part of a diet containing low-fat dairy (OR = 0.89; 95%CI: 0.80–0.98; P = 0.0005) was also associated with a lower prevalence of MetS. This finding was in contrast to observations indicating that low-fat dairy consumption was not associated with MetS risk, suggesting that milk-fat content mediates the health benefits of low-fat dairy. The PURE study also showed that higher dairy intake (≥2 servings/d vs 0) was associated with 11% (HR = 0.89; 95%CI: 0.82–0.97; P < 0.02) and 12% (HR = 0.88; 95%CI: 0.76–1.02; P < 0.01) lower incidence of hypertension and T2DM, respectively. Similar analyses also assessed the relationship of whole-fat and low-fat dairy foods on cardiometabolic risk. Although the consumption of whole-fat dairy alone exhibited similar directionality to lower cardiometabolic risk, the relationship was not statistically significant. Combined intake of whole-fat and low-fat dairy, however, corresponded with lower risks of hypertension (HR = 0.87; 95%CI: 0.79–0.96; P = 0.02) and diabetes (HR = 0.86; 95%CI: 0.73–1.02; P = 0.01). In another large-scale prospective study (n = 108 065 adults; 14.2 y follow-up), researchers also observed relationships between dairy-food intake and cardiometabolic risk.66 Greater consumption of nonfermented milk among men was associated with an increased risk of developing diabetes (HR = 1.17; 95%CI: 1.03–1.34; P = 0.018) and myocardial infarction (HR = 1.23; 95%CI: 1.10–1.37; P < 0.001). On the contrary, greater intake of butter, fermented milk, and cheese tended to correspond to a lower risk of diabetes and/or myocardial infarction. Those persons who consumed no dairy or chose lower-fat varieties of dairy had higher risks of diabetes, myocardial infarction, or stroke.66 Although the effect sizes of these observations were small, the findings support the concept that dietary inclusion of dairy fat, at least from certain foods, may reduce cardiometabolic risk.
In agreement with others,67,68 the totality of evidence from observational studies supports the concept that, at best, dairy foods have positive benefits on cardiometabolic risk and, at worst, neutral benefits. This perspective likely reflects the complexity of investigating whole foods on health outcomes, suggesting that stronger experimental approaches to assess dairy consumption could be helpful to resolve these questions. The odd-chain fatty acids C15:0 and C17:0 are highly abundant in dairy foods because they are derived from microbial biosynthesis in ruminants.69 These odd-chain fatty acids have been used as biomarkers of dairy intake to study the association of dairy intake with cardiometabolic health.67,70 The evidence supports an inverse relationship between cardiometabolic risk and odd-chain fatty acids, which reinforces the concept that higher-fat varieties of dairy foods may provide greater benefits than lower-fat varieties. For example, in a mouse model of NAFLD, supplementation of pentadecanoic acid (C15:0) reduced hepatic macrophage infiltration and decreased aspartate aminotransferase, suggesting that odd-chain fatty acids in dairy milk help protect against liver injury.71 A study in adults with NAFLD and age- and sex-matched healthy adults also showed that plasma phospholipid C17:0 and free fatty acids C15:0 and C17:0 were inversely related with glucose tolerance and liver fat.72 Similarly, findings of a meta-analysis of 16 prospective cohort studies identified that T2DM risk was lowest among people with the highest levels of C15:0 (HR = 0.80; 95%CI: 0.73–0.87), C17:0 (HR = 0.65; 95%CI: 0.59–0.87), and trans-16:1n-7.73 It is conceivable, therefore, that dairy fat, regardless of its source (eg, milk, cheese, yogurt), may reduce cardiometabolic risk.
Despite the growing number of reports that identify favorable health associations of dairy foods and dairy fat, recommendations of the Dietary Guidelines for Americans continue to emphasize the consumption of fat-free or low-fat dairy milk, yogurt, and cheese over full-fat varieties of dairy foods.33 This guidance aims to reduce excess dietary energy and saturated fat consumption to protect against CVD, obesity, and related disorders. In contradiction, evidence implicating dairy-food consumption in obesity risk is limited and inconsistent, and some studies actually identify favorable associations of full-fat dairy with adiposity in adults.74–78 Findings of a systemic review and meta-analysis of 10 prospective studies (> 46 000 children and adolescents; 3-y follow-up) also suggest that dairy consumption is not associated with childhood obesity risk, although the researchers did not distinguish between foods of varying dairy-fat content.79 Even when whole-fat dairy vs reduced-fat dairy (ie, some or all of the fat removed) was considered in a systematic review of studies in children aged 2–18 y (n = 29), it was concluded that cardiometabolic risk was not associated with whole-fat dairy consumption.80 Similar neutral effects were also concluded in an earlier meta-analysis of aggregated data in children and adolescents, although a positive benefit of dairy on adiposity was observed specifically among adolescents.81 In a cohort of preschool-age Latino children, a population with a disproportionate propensity for obesity, higher milk-fat consumption was associated with a lower risk of obesity (OR = 0.88; 95%CI 0.80–0.97; P = 0.01).82 Thus, although the rationale for limiting full-fat dairy consumption in the Dietary Guidelines for Americans is well intended to limit obesity, including that in children, it is unclear if the evidence substantiates this position. This perspective has received support in a review of cross-sectional (n = 43), longitudinal (n = 31), and randomized controlled studies (n = 20).83 The authors concluded that the majority of evidence suggests the risk of obesity was either unrelated to or inversely associated with dairy intake regardless of the type of milk or dairy product. Indeed, in < 10% of these studies was an unfavorable association observed between dairy foods and body weight.
Separate from excess dietary energy, the Dietary Guidelines for Americans recommends against full-fat dairy foods because of their high saturated-fat content.33 For example, 1 serving of fluid full-fat milk (240 mL) contains 4.5 g of saturated fat and 7.9 g of total fat per 148.8 kcal vs 83.3 kcal and <0.5 g of total fat per serving of nonfat milk.84 Such guidance arises in part from evidence originating from the Seven Countries Study and similar investigations85,86 that supports recommendations to limit total fat and saturated fat to < 30% and < 10% of total dietary energy, respectively.87,88 These recommendations, especially those related to saturated-fat consumption, are the subject of much debate89 and are inconsistent with findings of observational studies suggesting that all-cause mortality, CVD, stroke, and T2DM are unrelated to saturated-fat intake.90 Others also have reported that dietary saturated fat increases circulating low-density lipoprotein cholesterol (LDL-C) but noted that the increase often corresponds with an increase in large LDL particles that have a weaker relationship to CVD risk than do small LDL particles that have greater atherogenicity.91 In agreement with those observations, higher consumption of saturated fatty acids typically present in dairy foods is associated with fewer small LDL particles.92 Evidence from a rigorously controlled dietary intervention study in persons with MetS also examined how dietary saturated fat affects circulating saturated fatty acid levels.93 Participants were provided controlled diets that progressively increased in carbohydrate while the total and saturated fat content was decreased. Data showed that dramatic shifts in saturated-fat intake, from high saturated-fat intake during the low-carbohydrate phase to low saturated-fat intake during higher carbohydrate intake did not affect the proportion of total saturated fatty acids in any plasma lipid fractions. Rather, palmitoleic acid, a biomarker that has been reported to predict MetS and T2DM,94–99 increased during the high carbohydrate and low saturated-fat intervention phase but was lower during the low carbohydrate and high saturated-fat phase. Thus, evidence suggests that dietary saturated fats have limited detriment to health, at least when dietary carbohydrate is restricted, and that holistic dietary patterns need to be considered to fully assess the impacts of foods and their components.91 The latter are important to consider because the typical American diet is relatively high in both saturated fat and carbohydrate.29
The challenge of resolving the debate about dairy foods and dairy-fat consumption and cardiometabolic health stems from the lack of randomized controlled trials, which provide the strongest evidence to establish causal inferences.100 However, it is well recognized that not all diet- and health-related questions can be practically or ethically addressed through randomized controlled trials. Increased reliance, therefore, is placed on observations to formulate evidence-based dietary recommendations, especially prospective cohort studies in which dietary exposure can be assessed longitudinally before the onset of any clinical symptoms or disease pathology. Thus, although randomized controlled trials remain of critical importance, the preponderance of evidence from observational studies must be recognized. In this regard, it is difficult to conclude that dairy consumption adversely affects cardiometabolic health. To the contrary, evidence supports that it protects against the risk of poor cardiometabolic health. In part, these benefits appear to be influenced favorably by dairy fat.
Although removal of lipids, especially saturated fats, has been a primary focus of recommendations to consume nonfat or low-fat dairy foods,33 MPL (ie, phospholipids and sphingolipids) have gained attention for their cardiometabolic benefits.101 Importantly, lower-fat varieties of dairy foods often have decreased MPL content.55,102 It is plausible, therefore, that well-intended efforts to limit dairy-fat intake have an unintended consequence of limiting intake of health-promoting MPLs. Thus, in the following sections, we highlight bovine-derived MFGM and its MPL in relation to cardiometabolic health. Whenever possible, controlled studies in humans are emphasized (Table 111,103–113), and complementary evidence from preclinical models (Table 212–14,114–124) offers insight on the biological efficacy of MPLs.
Table 1.
Controlled studies investigating MPL on cardiometabolic outcomes in humans
| Reference | Population (no.) | Design | Duration | Treatment | Major outcomes |
|---|---|---|---|---|---|
| Overweight, postmenopausal women (n = 58) | Double-blind parallel | 4 wk | 0, 3, or 5 g of buttermilk PL |
|
|
| Patients with ileostomy (n = 4) | Double-blind crossover | 8 h postprandial | 0, 3, or 5 g of buttermilk PL with meal containing with 13C-triolein and 2H-cholesterol |
|
|
| Ohlsson et al (2010)103 | Healthy men (n = 20) | Single-blinded crossover | 8 h postprandial | High-fat meal + 975 mg buttermilk SL vs comparator | ↔ TG, TC, HDL-C, LDL-C, apoB/apoA1, insulin, glucose |
| Keller et al (2013)104 | Healthy women (n = 14) | Unblinded crossover | 10 d | 3 g or 6 g buttermilk PL or 6 g buttermilk PL + 2 g PSt |
|
| Ramprasath et al (2013)105 | Healthy men and women (n = 20) | Unblinded crossover | 14 d | Prescribed diet + 0 or 1 g milk SM |
|
| Overweight/obese older adult men (n = 62) | Double-blind parallel | 8 wk | 0 or 2 g butter serum PL |
|
|
| Overweight/obese older adult men (n = 57) | Double-blind parallel | 7 wk | 3 g MPL or 2.8 g soy PL |
|
|
| Rosqvist et al (2015)107 | Overweight, older adults (n = 57) | Single-blind parallel | 8 wk | 40 g whipping cream (intact MFGM with 19.8 mg PL) or 40 g butter oil (1.3 mg PL) |
|
| Beals et al (2019)108 | Overweight/obese men and women (n = 36) | Double-blind crossover | 6 h postprandial | Whipping cream + MFGM (PL not reported) |
|
| Demmer et al (2016)109 | Overweight/obese men and women (n = 36) | Double-blind crossover | 6 h postprandial | Palm oil + MFGM |
|
| Conway et al (2013)110 | Men and women with high LDL-C levels (n = 34) | Double-blind crossover | 4 wk | Buttermilk (187.5 mg PL) or control (34.6 mg PL) |
|
| Conway et al (2014)111 | Men and women with high LDL-C levels (n = 34) | Double-blind crossover | 4 wk | Buttermilk (187.5 mg PL) or control (34.6 mg PL) |
|
| Ten Bruggencate et al (2016)112 | Healthy young adults (n = 58) | Double-blind parallel | 4 wk | Daily soy + MFGM (0 or 3.2 g PL) + Escherichia coli challenge at week 2 |
|
| Ohlsson et al (2010)113 | Patients with ileostomy (n = 6) | – | 8–9 h | 250 mg SM | ↑ C22:0 SM, C23:0 SM, C24:0 SM, sphingosine, and ceramide in ileostomy content compared with presupplementation ileostomy content |
Abbreviations: ACE, angiotensin-converting enzyme; ALT, alanine aminotransferase; ANG II, angiotensin II; apoA, apolipoprotein A; apoB, apolipoprotein B; AST, aspartate transaminase; BW, body weight; CD14, cluster of differentiation; CFAII, colonization factor antigen II; CMRF-C, chylomicron-rich fraction cholesterol; CMRF-TG, chylomicron-rich fraction triglyceride; CRP, C-reactive protein; EPHX2, epoxide hydrolase 2; ER, endoplasmic reticulum; FSR, fractional synthesis rate; GGT, γ-glutamyl transferase; GI, gastrointestinal; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment of insulin resistance; iAUC, incremental area under the curve; Ig, immunoglobulin; IL, interleukin; LBP, lipopolysaccharide-binding protein; LDL-C, low-density lipoprotein cholesterol; LPS, lipopolysaccharide; LTBR, lymphotoxin β receptor; MAP, mean arterial pressure; MCP-1, monocyte chemoattractant protein-1; MFGM, milk fat globule membrane; MPL, milk phospholipid; MUFA, mono-unsaturated fatty acid; PCSK9, protein convertase subtilisin kexin-9; PL, phospholipid; PSt, plant sterol supplementation; SAA, serum amyloid A; SBP, systolic blood pressure; SCFA, short-chain fatty acid; sICAM-1, soluble intercellular adhesion molecule-1; TC, total cholesterol; TG, triglyceride; tHcy, total homocysteine; TNF-α, tumor necrosis factor-α; VLDL-C, very-low-density lipoprotein cholesterol; WC, waist circumference.
Table 2.
Controlled studies investigating MPL on cardiometabolic outcomes in rodents
| Reference | Model (no.) | Duration | Treatment | Major outcomes |
|---|---|---|---|---|
| Millar et al (2020)114 | Male LDLr−/− mice (n = 45) | 14 wk | HFD + 0, 1% or 2% (w/w) MPL |
|
| Milard et al (2019)115 | Male C57Bl/6 mice (n = 45) | 8 wk | LFD vs HFD + 0, 1.1%, or +1.6% (w/w) butter serum MPL |
|
| Milard et al (2019)116 | Male C57BL/6 mice (n = 40) | 4-h | MSM (5 or 10 mg) or MPL (5 or 10 mg) |
|
| Zhou et al (2019)12 | Male ob/ob mice (n = 18) | 2 wk | HFD vs HFD + milk GG (0.2 g/kg) or MPL (10 g/kg of diet) |
|
| Norris et al (2017)117 | Male C57BL/6 mice (n = 52) | 10 wk | LFD vs HFD + 0, MSM (0.1%), or egg sphingomyelin (0.1%) |
|
| Lecomte et al (2016)118 | Male C57BL6 mice (n = 22) | 8 wk | LFD vs HFD + 1.2% soy PL or 1.2% MPL |
|
| Wat et al (2009)119 | Male C57BL6 mice (n = 40) | 8 wk | Control diet (0 or 1.2% milk PL) or HFD (0 or 1.2% milk PL) |
|
| Kamili et al (2010)120 | Male C57BL/6 mice (n = 20) | 3,5, or 8 wk | HFD + 0, 1.2% milk PLs |
|
| Mathiassen et al (2015)121 | Female BALB/C mice (n = 36) | 30, 60, or 120-min | Soy PLs or milk PLs with 3H-inulin |
|
| Norris et al (2016)13 | Male C57BL/6J mice (n = 30) | 4 wk | HF, + 0.25% milk SM, or + 0.25% egg SM |
|
| Lecomte et al (2015)122 | Female Swiss mice (n = 7 per group) | 1, 2, or 4-h | Water and oil emulsion enriched with 5.7 mg soy PLs or milk PLs |
|
| Eckhardt et al (2002)123 | Male C57L/J mice (n = 6) | 4 d w/crossover | Control diet or milk PL + intragastric bolus of 0.15 mL medium-chain triglyceride containing 14C-cholesterol and 3H-sitostanol at day 4 |
|
| Li et al (2020)124 | Male C57BL/6 mice (n = 40) | 8 wk | HFD + 100 mg, 200 mg, or 400 mg/kg bodyweight MFGM |
|
| Norris et al (2017)14 | Male C57BL/6J mice (n = 28) | 10 wk | HFD or + 0.1% milk SM |
|
Abbreviations: ABGC5, ATP binding cassette subfamily G member 5; Acaa2, acetyl-CoA acyltransferase 2; Acacb, acetyl-CoA carboxylase β; Acat2, acetyl-CoA Acetyltransferase 2; ACOX, acyl-CoA oxidase 1; Adgre1, adhesion G protein-coupled receptor E1; ALT, alanine aminotransferase; Apob, apolipoprotein B; AST, aspartate transaminase; CCL2, C-C motif chemokine ligand 2; ccl4, macrophage inflammatory protein-1 β; cd36, cluster of differentiation 36; CE, cholesteryl ester; Cpt1b, carnitine palmitoyltransferase-1b; CXCL1, C-X-C motif chemokine ligand 1; FABP2, fatty acid binding protein 2; FATP4, fatty acid transport protein; FITC-dextran, fluorescein isothiocyanate-dextran; GG, milk gangliosides; GGT, γ-glutamyl transferase; GLUT4, glucose transporter 4; HDL-C, high-density lipoprotein C; HFD, high-fat diet; HMGCoA, 3-hydroxy-3-methylglutaryl-CoA reductase; IFN-γ, interferon γ; IL, interleukin; LFD, low-fat diet; LXRa, liver X receptor α; MIP-1β, macrophage inflammatory protein-1 β; MPL, milk phospholipid; MSM, milk sphingomyelin; MTTP, microsomal triglyceride transfer protein; NEFA, nonesterified fatty acid; NPC1L, Niemann-Pick C1 like 1; PGC-1α, peroxisomal proliferator-activated receptor coactivator-1α; PL, phospholipid; Pla2g2, phospholipase A2g2; PPARα, peroxisome proliferator-activated receptor-α; PRDM16, PR domain-containing 16; Reg3y, regenerating islet-derived protein 3 gamma; Scd1, stearoyl-CoA desaturase; sCd14, soluble cluster of differentiation 14; SM, sphingomyelin; SR-B1, scavenger receptor class B type 1; SREBP1c, sterol regulatory element-binding protein 1c; Srebp2, sterol regulatory element-binding protein 2; TC, total cholesterol; TG, triglyceride; TNF-α, tumor necrosis factor-α; UCP1, uncoupling protein 1; WAT, white adipose tissue; w/w, weight per weight; ZO-1, zonula occluden-1.
CARDIOMETABOLIC BENEFITS OF MFGM-DERIVED PHOSPHOLIPIDS
The MFGM originates from the apical surface of mammary epithelial cells and comprises a unique triple phospholipid and cholesterol layer embedded with proteins and glycoproteins that surround secreted milk-fat droplets.125 The genes that synthesize MFGM are conserved across species and the composition of MFGM differs little between species. The MPL content accounts for 1% of total milk lipid, with approximately one-third each from SMs, phosphatidylcholines, and phosphatidylethanolamines.126 MFGM is present in most dairy foods, but churned buttermilk is especially rich in the membrane fraction.10 Churning of milk fat disrupts the membrane surrounding the fat globules and results in the coalescence of free fat that ultimately yields butter. In contrast, buttermilk is the aqueous phase (ie, MFGM-containing portion). Although various MFGM constituents (eg, polar lipids, gangliosides, proteins/enzymes) are reported to have health-promoting bioactivities,101 we focus in this review on the cardioprotective activities of MPL, especially milk SM (MSM).
Lipid digestion and absorption
Dietary polar lipids are recognized for their function as natural emulsifiers in foods, with as much as 90% of the world market using soy lecithin.11 However, MPLs are an alternative natural source of emulsifiers and notable for their high MSM content, which represents ∼25% of total MPL. The chemical properties of MPLs make them highly effective to limit intestinal uptake of certain lipids,123,127 with evidence indicating MSM but not egg phosphatidylcholine can disrupt micellar partitioning and absorption of cholesterol.123 This finding supports that full-fat dairy foods, with their higher MPL content,55 may reduce cardiometabolic risk by limiting dietary and/or biliary cholesterol absorption in the intestine.
The influence of polar lipids on dietary lipid absorption has been studied in lymph-cannulated rodents in which lipid emulsions were introduced to the upper duodenum via an indwelling catheter.128 These studies have revealed that infusion of a lipid emulsion containing phosphatidylcholine (as either 1,2-dipalmitoyl phosphatidylcholine or 1,2-dilinoleoyl phosphatidylcholine) inhibits the absorption of fat-soluble vitamin E (α-tocopherol) by ∼50%, whereas the absorption of triolein (ie, triglyceride) increased by 42%–43%. Similar studies in this model system also show that 1,2-dioleoyl phosphatidylcholine, but not 1-oleoyl-2-hydroxy- phosphatidylcholine (lysophosphatidylcholine), reduced the absorption of both α-tocopherol and cholesterol. These findings support the concept that polar phospholipids affect the absorption of certain lipids. However, whether MPLs exert a similar effect was not evaluated in these studies. Indeed, in contrast to the polar lipids of nondairy origin that were used in this study,128 milk phospholipids have a different fatty acid composition, with a saturated fatty acid (C14:0–18:0) typically present at the sn-1 position and an unsaturated fatty acid (C18:1-C18:2) at sn-2.51
Dietary sphingolipids in the Western diet consist mainly of SM that originates from dairy foods (∼25% of total MPLs), meat (∼5%–10% of total polar lipid), and eggs (∼2% of total polar lipid).129 Of interest is that the SM species in dairy differ from those in other foods. MSM has fatty acids of very long chain lengths (C22:0-C24:0) compared with egg SM, which commonly has palmitic acid (C16:0).13 The distribution of sphingoid bases (d16:0 to d19:0) is also more varied in MSMs and likely contributes to their favorable bioactivity to differentially interact with and desorb cholesterol from cellular membranes.130 The fatty acid composition of MSMs also more greatly inhibits intestinal lipid absorption than that of egg SM, which is potentially due to stronger hydrophobic interactions in the intestinal lumen by MSM.127
Structural differences between egg SM and MSM prompted studies127 in the aforementioned lymph-cannulated rat model.128 Interestingly, MSM compared with egg SM decreased the absorption of cholesterol, α-tocopherol, and triolein while reducing lymphatic output of phosphatidylcholine and SM.127 These effects were attributed partly to an intraluminal interaction in which hydrogen bonding occurs between cholesterol and SM to decrease micellar solubilization and/or promote a slower transfer of cholesterol from the micellar matrix to the enterocyte. Also, MSM may have limited pancreatic lipase activity, consistent with radiotracer studies in vitro and in mice indicating that MPL attenuates free fatty acid release from emulsified triglyceride.121 The differential inhibition of MSM and egg SM on lipid absorption is likely due to their unique fatty acid chain lengths such that MSM has a greater abundance of very-long-chain saturated fatty acids (22:0, 23:0, and 24:0; 66% in total) in lieu of C16:0 that is highly abundant in egg SM (87%). Nonetheless, this lymph-cannulated rodent model provided strong evidence to support that MSM is a potent absorptive inhibitor of cholesterol and other lipids, suggesting that chronic MPL ingestion could have a cardiometabolic benefit.
Lipid-lowering effects in circulation and liver
In agreement with the cardiometabolic health benefits of milk phospholipids that decrease intestinal lipid absorption, chronic supplementation with milk phospholipids also attenuated hepatic cholesterol and triglyceride accumulation in preclinical rodent models.131 This was demonstrated in mice provided a high-fat diet containing 1.2% cow’s milk phospholipid.120 Others have reported similar effects of MSM or MPL in reducing hepatic triglyceride and/or cholesterol ester in LDL-receptor knockout mice or wild-type mice fed a high-fat diet.13,114,117 Evidence for an intraluminal effect was provided by data showing greater recovery of fecal 14C-cholesterol and decreased recovery of hepatic 14C-cholesterol. Hepatic cholesterol and triglyceride were also correlated with hepatic 14C-cholesterol (P < 0.001), whereas hepatic cholesterol and triglyceride were inversely related to fecal 14C-cholesterol (P < 0.01–0.05). It was also reported that milk-derived ceramide levels in liver tended to be lower despite substantially higher levels of dietary and fecal ceramides in mice supplemented with milk phospholipids. These findings suggest that milk phospholipids protect against liver steatosis, an asymptomatic but potentially debilitating cardiometabolic disorder,23 consistent with a mechanism of limiting intestinal lipid absorption. Although liver steatosis was not evaluated, findings of a placebo-controlled trial in middle-aged adults showed that MFGM alleviates hyperlipidemia by limiting lipid absorption.110 Adults provided 45 g/d buttermilk for 4 weeks had lower total cholesterol (P = 0.019) and LDL-C (P = 0.057) levels. The surrogate biomarker of intestinal cholesterol absorption, β-sitosterol, was also correlated with circulating total cholesterol and LDL-C. Whether these cardiometabolic benefits are attributed entirely to MPL could not be established in this study. This is because buttermilk closely reflects the composition of MFGM, including MPL and membrane proteins (eg, MFG-EGF-8); the latter are also known to exhibit health-promoting bioactivities.132,133
Nonetheless, these protective effects against hypercholesterolemia are consistent with earlier evidence in vitro showing that buttermilk limits cholesterol micellar solubility134 and recent investigations in humans showing that MPLs decrease cholesterol absorption.11 Thus, evidence in humans and rodents supports that MPLs promote cardiometabolic health by lowering levels of circulating and hepatic cholesterol, likely due to a gut-level effect that limits lipid absorption. Similar benefits of MPLs to reduce hepatic triglyceride are also apparent in rodents, but these health effects have yet to be studied adequately in humans.
Lipid digestion and lipemia
Studies in mice and in vitro have examined whether the intraluminal emulsification activities of MPLs affect postprandial lipemia and lipid digestion, respectively.122 Mice gavaged with an emulsion stabilized with either MPL or soy polar lipids, which differ primarily in SM content (29.9% vs 0%), had a more rapid increase, but more rapid clearance, of plasma triglyceride in response to MPL; similar effects occurred for plasma nonesterified fatty acids. Plasma ApoB48 was also initially higher at 1 hour after MPL administration compared with soy polar lipids, but this phenomenon was the converse by 4 hours postadministration of polar lipids. Similarly, duodenal Apob mRNA expression was lower at 4 hours post-gavage among mice receiving MPLs, whereas a main effect for emulsion type indicated that Mttp expression was higher in response to MPL, with a specific between-treatment effect observed at 1 hour post-gavage. In agreement, chylomicron lipid content was higher at 1 hour after MPL administration but was significantly lower by 4 hours compared with the soy polar lipid treatment. However, chylomicron particle size among mice indicated MPLs were larger and there was a greater proportion of 24:1 ceramide, which has been suggested to be protective against MetS.135
To help explain these observations in mice,122 in vitro digestion studies assessed the differential effects of MPL vs soy polar lipids on triglyceride digestive hydrolysis. No difference between polar lipid treatments occurred during the gastric phase of digestion, whereas MPLs during intestinal digestion increased triglyceride hydrolysis. The intestinal-level benefits of MPL are attributed at least in part to MSM. This is consistent with SM being a precursor to ceramide formation following intestinal digestion, and that the hydrolysis of ceramides promotes the production of large chylomicrons.136 Interestingly, MPL-mediated triglyceride hydrolysis also may depend on the presence of milk glycerophospholipids, based on evidence that the addition of MSM to soy lecithin emulsions inhibits pancreatic lipase activity and combined hydrolysis from gastric and pancreatic lipases.121 Taken together, MPLs uniquely protect against postprandial lipemia compared with soy polar lipids, which is consistent with a mechanism of inhibiting chylomicron assembly; increasing their particle size, which would be expected to enhance their clearance137; and increasing triglyceride hydrolysis in the intestinal lumen. These findings also help explain that dietary SM and ceramides of MPL reduce fasting triglyceride of rodents.119,138 Similarly, 4-week supplementation of an MPL-rich emulsion, but not a formula containing egg polar lipids, prevented an increase in plasma triglyceride levels in humans,139 and phospholipid-rich buttermilk ingestion decreased fasting triglyceride levels in a placebo-controlled study.110 However, in a separate study in humans in which the effect was compared of MPL and egg polar lipids on postprandial hypertriglyceridemia following a high-fat breakfast challenge, researchers found no between-treatment effects.103 Two studies of parallel design conducted with men who consumed either drink enriched with 2 g of MPL compared with a control for 8 weeks, or test drinks formulated with MPL vs soy polar lipids for 7 weeks, also showed no effects on circulating lipids, apolipoproteins, biomarkers of inflammation, or insulin resistance.106 These studies contradict the findings by Rosqvist et al,107 who showed in an 8-week intervention that supplementation of MFGM with dairy fat prevents the increase in plasma lipids (namely, total cholesterol and LDL-C) and the ratio of apolipoprotein B to apolipoprotein A-I that are otherwise observed in response to butter oil. These findings suggest the underlying food matrix of dairy foods must be carefully considered when evaluating their cardiometabolic benefits.
Others have examined the influence of MFGM on postprandial metabolism relative to inflammatory responses consistent with the recognition that the daily accumulation of metabolic and inflammatory insults contributes incrementally to CVD risk.140,141 Demmer et al109 conducted a randomized, double-blinded, crossover trial to examine whether MFGM supplementation in overweight and obese middle-aged adults would attenuate postprandial inflammation and hyperlipidemia otherwise induced by a high-saturated fat meal (high in palmitate). MFGM in the high-fat meal challenge lowered total cholesterol and LDL-C levels but increased triglyceride levels while reducing insulin over the 6-hour postprandial period. Postprandial soluble intercellular adhesion molecule-1 levels also were lowered by MFGM, whereas the levels of the anti-inflammatory cytokine interleukin (IL)-10 were increased; numerous other pro-inflammatory cytokines were unaffected (eg, tumor necrosis factor (TNF)-α, IL-8). Although these findings support MPLs as limiting adverse metabolic and inflammatory effects caused by a high-fat meal, the MFGM ingredient used in this study also contains membrane proteins that potentially contribute to the observed benefits, consistent with a report of anti-inflammatory effects of MFG-EGF-88 in a colitis model.132 Additional studies are warranted to define the independent benefits of MPL and/or its specific lipid species on lipemia.
Inflammation and gut barrier permeability
Cardiometabolic health, including obesity and insulin resistance, is linked to gut health and the resistance of the intestinal barrier to permit the translocation of endotoxins that provoke Toll-like receptor-4/nuclear factor (NF)-κB inflammation.142–144 In a neonatal rodent model, buttermilk-derived MPL improved gut barrier function by downregulating intestinal inflammation otherwise induced by endotoxin-Toll-like receptor-4-NF-κB.145 Membrane-rich milk fat also protected mice against lipopolysaccharides-induced impairments in gut barrier integrity.146 Likewise, studies in vitro using cell-based assays on neutrophils or monocytes suggested promise for select phospholipid- or ganglioside-rich fractions of MFGM to attenuate inflammatory responses (eg, neutrophil elastase activity, superoxide production, nitric oxide production, IL-1β release, cyclooxygenase-1 and -2 activities).147
Milard et al116 examined the effects of MSM and MPL on gut physiology. MSM in human epithelial intestinal Caco-2/TC7 cells increased expression of the tight junction proteins occludin and zonula occluden-1 without affecting claudin-1. Analogous studies with an MPL-rich ingredient did not show a similar benefit. Follow-up studies specifically with MSM showed no benefit on transepithelial electrical resistance or cellular permeability to chemical probes, suggesting no functional improvement in gut integrity. MSM also did not affect the secretion of IL-6, IL-17, IL-22, TNF-α, or MCP-1, but it unexpectedly increased the expression and secretion of IL-8. These findings were extended by showing that IL-8 treatment causes tight junction protein overexpression, suggesting that MSM regulates barrier function through an indirect mechanism. Parallel studies in C57BL6 mice supported this mechanism, consistent with MSM increasing the expression of homologs of IL-8. Additional study is warranted to better define the indirect “pro-inflammatory” activities of MSM on gut barrier function.
Researchers hypothesized that in leptin-deficient (ob/ob) mice, which are obese in association with impaired gut barrier integrity and elevated circulating endotoxin,148,149 MPL (provided as phospholipids or gangliosides) would protect against gut barrier function in association with inhibiting inflammation and liver steatosis.12 Treatment with either MPL did not affect 2 separate indices of gut permeability nor did either MPL attenuate circulating endotoxin. Tight junction protein expression was largely unaffected by either treatment with the exceptions that ganglioside supplementation increased colonic occludin, and supplementation with ganglioside or phospholipid decreased jejunal zonula occluden. Hepatic triglyceride was also unaffected, but phospholipid supplementation decreased hepatic cholesterol ester. Plasma cytokines and adipokines were also unaffected, except that levels of circulating IL-6 increased in response to phospholipid supplementation. These unexpected outcomes were potentially attributed to the severity of preexisting obesity in ob/ob mice and/or they indicate that intact leptin signaling is required to observe the benefits of MPLs. Indeed, MSM supplementation in wild-type mice provided a high-fat diet prevented diet-induced increases in serum endotoxin levels.13 However, this protective effect occurred without any improvement of gut barrier permeability or expression of intestinal tight junctions. These findings support that MSM reduced availability of gut-derived endotoxins from the microbiota, as discussed in the next section.
Gut microbiota and cardiometabolic risk
The gut microbiota is recognized to influence cardiometabolic health. To explore this, preclinical and clinical models were used in studies to define the potential prebiotic and/or antimicrobial activities of MPLs that alleviate cardiometabolic risk. C57BL6 mice were provided a high-fat diet supplemented with milk phospholipids (primarily as phosphatidylcholine and phosphatidylethanolamine), milk sphingolipids (primarily as MSM), or total MPLs.150 Sphingolipid supplementation tended to reduce hepatic triglyceride biosynthesis (P = 0.06), whereas supplementation of phospholipids or MPLs significantly (P < 0.05) decreased hepatic de novo hepatic fatty acid biosynthesis. These effects occurred without any notable changes in gut microbiota composition, and the few, significant, pair-wise associations between bacterial populations and mouse phenotype (eg, body mass, liver mass, hepatic triglyceride or cholesterol, biomarkers of lipogenesis) were not consistent across treatments.150 These findings suggest that unique species of MPL function differentially to regulate hepatic lipogenesis and protect against metabolic dysfunction but independently of any improvements in gut microbial community structure or function. Indeed, overweight, postmenopausal women (n = 58) who were provided MPL supplementation for 4 weeks in a parallel study design (0, 3, or 5 g/d; ∼25% as SM) had no notable changes in major gut bacterial populations or fecal short-chain fatty acids.11 The limited dietary control and lack of crossover design that can assess within-subject effects could have strengthened the approach to potentially observe treatment effects. Nonetheless, MPLs at 5 g/d decreased the number of intestine-derived chylomicron particles in association with favorable changes in fasting and postprandial total cholesterol concentrations, the ratio of total cholesterol to high-density lipoprotein cholesterol, and the ratio of apolipoprotein B to apolipoprotein A1. Consistent with the premise that MPLs can decrease cholesterol absorption, fecal coprostanol, the gut-derived metabolite of cholesterol, was increased. A complementary study also showed that the ingestion of MPLs by patients with ileostomy decreased cholesterol absorption,11 a finding that agrees with those indicating dietary MSM is incompletely absorbed and inhibits cholesterol absorption.113 Of particular interest are the secondary analyses performed with obese postmenopausal women that showed MPL decreased atherogenic species of SM (C16 + 18 SM) and ceramide (C24:1) species in the serum,151 and that C16 + 18 SM was correlated with favorable changes in total cholesterol, LDL-C, and ApoB levels. Despite high-dose supplementation of SM from MPLs, SM and ceramide concentrations in intestine-derived chylomicrons were decreased, whereas their levels in fecal contents increased. This suggests that an intestinal-level mechanism involving SM metabolism was responsible for these cardiometabolic benefits. These studies in rodents and humans also suggest that metabolic benefits from MPLs can occur independently of prebiotic effects on gut microbiota to limit hepatic lipogenesis and cholesterol absorption. However, recent studies in vitro indicate that MPLs enhance the cellular adhesion of lactic acid bacteria to Caco-2 cells,152 suggesting that researchers should consider whether MPLs selectively influence mucosa-associated and luminal microbial communities that are distinct and differentially affected by environmental exposures.153
Consistent with evidence that MPLs protect against dyslipidemia, studies in LDL-receptor knockout mice were conducted to examine their potential to ameliorate atherosclerosis development in relation to altering gut microbial structure.114 Supplementation of MPL at 1% or 2%, which corresponds to 0.2% or 0.4% MSM, as part of a high-fat and high-cholesterol atherogenic diet alleviated diet-induced increases in serum total cholesterol and very-low-density lipoprotein cholesterol and LDL-C; these effects were more pronounced at the higher MPL dose. Strikingly, MPLs (2%) reduced atherosclerotic plaque lesions in association with reduced markers of inflammation (ie, circulating CCL2, hepatic CCL4, hepatic Adgre1 [P = 0.08], and adipose [CCL2]). These anti-atherogenic benefits corresponded with putative improvements in gut microbiota composition, specifically increased abundances of Bacteroidetes, Actinobacteria, and Bifidobacterium, and a decreased abundance of Firmicutes. Thus, the findings of studies contrasted with those of earlier preclinical and clinical studies by suggesting that prebiotic activities of MPLs help to protect against CVD.
A study by Norris et al13 further supports that MPLs exert cardiometabolic benefits by favorably altering populations of commensal and pathogenic bacteria while protecting against dysfunctional lipid metabolism implicated in NAFLD. In wild-type mice provided a high-fat diet (anhydrous milk fat) alone or supplemented with MSM or egg SM (0.25% weight per weight), serum total cholesterol and triglyceride levels increased in response to egg SM, whereas total cholesterol decreased without any changes in circulating triglyceride in response to MSM. Notably, hepatic triglyceride levels increased with egg SM supplementation in contrast to the favorable decreases induced by MSM that were associated with lowered expression of stearoyl-CoA desaturase-1. Additional gene expression measures suggested that cholesterol depletion occurred in intestinal and liver tissue. Of interest was that only MSM supplementation improved metabolic endotoxemia, but this was not explained by changes in gut permeability. Rather, MSM decreased total gram-negative bacteria (ie, endotoxin-containing) including Tenericutes, while increasing commensal populations (eg, Actinobacteria, Bifidobacterium) that have been suggested to have cardiometabolic benefits. On the basis of these findings, the antimicrobial activities of MSM likely decreased pyrogenic bacteria but apparently did not improve gut barrier permeability to limit endotoxin translocation. It is also possible that the known effects of MSM on cholesterol absorption and chylomicron assembly also limited chylomicron-dependent endotoxin absorption. More study in this area is needed to clarify the mechanisms by which MSM protects against endotoxemia-associated NAFLD and whether these benefits can be translated to humans.
Adiposity and inflammation
To our knowledge, no strong evidence in humans supports that MPL lowers the risk for obesity. However, findings from preclinical rodent models show promise that the anti-inflammatory activities of MPL may help reduce adiposity. For example, dietary supplementation of egg SM, but not MSM, increased adiposity in mice fed a high-fat diet.13 Similar studies with MPL and soybean phospholipid have been conducted in mice fed a high-fat diet for 8 weeks.118 Increased adipose mass and larger adipocytes were observed in mice supplemented with soybean phospholipid but not MPLs; the soybean phospholipid treatment also exacerbated hepatic triglyceride content. Interestingly, the worsening of obesity due to soybean phospholipid supplementation corresponded with increased expression levels of adipose pro-inflammatory markers (eg, Tnfα, Mcp-1, F4/80, lipopolysaccharide-binding protein), whereas none of these genes was upregulated by MPL supplementation in contrast to the lowered level of adipose Cd68. It is possible that MPLs are not anti-obesigenic per se but instead have a role to protect against dietary insults that provoke adiposity. However, recent studies have shown near dose-dependent effects of MPL at 1.1% and 1.6% to protect against body mass gain in mice fed a high-fat diet.115 Plasma pro-inflammatory proteins were largely unaffected by MPLs, but hepatic expression of the macrophage marker F4/80 tended to be downregulated (P = 0.06), and populations of gut bacteria of metabolic interest were altered by MPL. However, it is noteworthy that MSM blocks lipopolysaccharide-induced increases in pro-inflammatory gene expression in RAW264.7 macrophages, and these anti-inflammatory effects are lost when hydrolysis of MSM is inhibited.14 A separate line of investigation in obese mice and in cultured cells showed that MFGM, likely mediated by its phosphatidylcholine content, protects against obesity by inducing adipose browning.124 Collectively, emerging evidence in this area suggests that MPLs may limit obesity risk by lowering adipose inflammation, but mechanistic studies are needed to define the mechanisms by which MFGM- and MPL-enriched dairy products protect against obesity and whether the effects are mediated by the gut microbiota. Clinical validation is also required to translate these promising cardiometabolic benefits to improve human health.
Conclusion
This review was conducted to summarize the benefits of milk fat, specifically those attributed to MPLs, relative to their potential dietary role in managing the growing trends of poor cardiometabolic health. Although higher-quality dietary patterns have potential to thwart cardiometabolic risk, a critical need exists to establish the mechanisms by which bioactive food components regulate pathogenic responses. Bovine milk is recognized for its nutrient-rich profile, but debate continues about whether its total fat content adversely affects cardiometabolic health. Much of this controversy is attributed to the significant body of evidence derived from observational studies that can only establish associations. This supports a need for controlled studies, especially in humans, that are designed to establish causality. In addition, overall dairy consumption has declined in the United States, and the limited milk that is consumed is of lower-fat varieties that have reduced MPL content.154 This is of potential concern in terms of cardiometabolic health, because the MPL content, especially MSM, has demonstrated benefits in humans, with mechanistic support from preclinical rodent models, to protect against hypercholesterolemia by influencing intraluminal emulsions and limiting intestinal cholesterol absorption (Figure 3).
Figure 3.
Summary of cardiometabolic activities of milk polar lipids (MPLs) in humans and rodent models. MPLs consisting of LacCer, GluCer, SM, and glycerophospholipids (PE, PC, PI, and PS) and several membrane-associated proteins are present in the MFGM trilayer. PC, SM, and PS are generally localized to the outer membrane; PE, PC, SM, and PS are more enriched in the inner membrane; and PI and PS compose the majority of the inner monolayer.155 MPLs, especially SM, were noted in this review to have significant cardioprotective activities; the appearance of a rodent and/or human image in the figure denotes model system-specific benefits of MPL on cardiometabolic health outcomes. The most compelling evidence in humans, and supported by rodent studies, indicates that MPLs reduce circulating cholesterol levels by intraluminal emulsification activities that limit intestinal cholesterol absorption. Although studies in humans are lacking, evidence from rodent models support MPLs as improving liver health and gastrointestinal health, potentially involving prebiotic and/or antimicrobial activities on gut microbiota. Although relatively more limited in study regardless of model system, MPL in rodents reduces adiposity, possibly through a mechanism involving the browning of adipose tissue. Note: Schematic representation of MFGM is not drawn to scale. Abbreviations: ADPH, adipophilin; BTN, butyrophilin; CD36, cluster of differentiation 36; CHOL, cholesterol; FABP, fatty acid–binding protein; GPL, glycerophospholipid; GluCer, glucosyl ceramide; LacCer, lactosyl ceramide; LDL-C, low-density lipoprotein cholesterol; MFG-EGF8, milk fat globule–epidermal growth factor 8 protein; MFGM, milk fat globule membrane; MPL, PC, phosphatidylcholine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; PS, phosphatidylserine; SM, sphingomyelin; TJP, tight junction protein; VLDL-C, very-low-density lipoprotein cholesterol; XO, xanthine oxidase
At least in rodents, MPLs regulate diverse cardiometabolic responses. These include gut-level benefits to exert prebiotic and antimicrobial activities on gut microbiota as well as limiting metabolic endotoxemia, inflammation, and NAFLD risk. However, such benefits in humans have not been established, due to a lack of study, and reflect a need for controlled clinical studies. Efforts will require large-scale intervention studies, ideally with prescribed basal diets, to isolate the independent effects of MPLs on gut bacteria community structure and health-promoting functions. Such studies would also be expected to yield considerable inter-individual variability. Thus, the integration of multi-omics approaches may help define personalized approaches for effective nutritional management of cardiometabolic risk.156 The needed rigorous experimental approaches, especially randomized controlled trials, coupled with omics workflows may also help answer a critical question: do the emerging and confirmed cardiometabolic benefits of MPL outweigh any unresolved concern about the higher saturated fat and energy content of full-fat dairy milk?
Acknowledgments
Funding. This work was supported by grants to R.S.B. from the National Dairy Council, the US Department of Agriculture (USDA) HATCH program (grant OHO01452-MRF), and the Ohio Agricultural Research and Development Center at The Ohio State University. C.N.B. received support from USDA-NIFA (grant CONS2020-07703) and USDA-HATCH (grant CONS01011).
Declarations of Interests. M.T.-G. is employed by the National Dairy Council. C.N.B. and R.S.B. have received grant support and honorarium for scientific consulting activities from the National Dairy Council. A.P. has no interests to declare.
Author contributions. R.S.B. and A.P. drafted the manuscript. All authors contributed to critically reviewing and revising the final manuscript.
References
- 1. Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. [DOI] [PubMed] [Google Scholar]
- 2. Standl E, Khunti K, Hansen TB, et al. The global epidemics of diabetes in the 21st century: current situation and perspectives. Eur J Prev Cardiol. 2019;26:7–14. [DOI] [PubMed] [Google Scholar]
- 3. Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Virani SS, Alonso A, Benjamin EJ, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. [DOI] [PubMed] [Google Scholar]
- 5. Drewnowski A. The Nutrient Rich Foods Index helps to identify healthy, affordable foods. Am J Clin Nutr. 2010;91:1095S–1101S. [DOI] [PubMed] [Google Scholar]
- 6.Dietary Guidelines Advisory Committee. Scientific Report of the 2020 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Agriculture and the Secretary of Health and Human Services. Washington, DC: U.S. Department of Agriculture, Agricultural Research Service; 2020.
- 7. Scrafford CG, Bi X, Multani JK, et al. Health care costs and savings associated with increased dairy consumption among adults in the United States. Nutrients. 2020;12:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hirahatake KM, Astrup A, Hill JO, et al. Potential cardiometabolic health benefits of full-fat dairy: the evidence base. Adv Nutr. 2020;11:533–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lordan R, Tsoupras A, Mitra B, et al. Dairy fats and cardiovascular disease: do we really need to be concerned? Foods. 2018;7:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dewettinck K, Rombaut R, Thienpont N, et al. Nutritional and technological aspects of milk fat globule membrane material. Int Dairy J. 2008;18:436–457. [Google Scholar]
- 11. Vors C, Joumard-Cubizolles L, Lecomte M, et al. Milk polar lipids reduce lipid cardiovascular risk factors in overweight postmenopausal women: towards a gut sphingomyelin-cholesterol interplay. Gut. 2020;69:487–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhou AL, Ward RE.. Milk polar lipids modulate lipid metabolism, gut permeability, and systemic inflammation in high-fat-fed C57BL/6J ob/ob mice, a model of severe obesity. J Dairy Sci. 2019;102:4816–4831. [DOI] [PubMed] [Google Scholar]
- 13. Norris GH, Jiang C, Ryan J, et al. Milk sphingomyelin improves lipid metabolism and alters gut microbiota in high fat diet-fed mice. J Nutr Biochem. 2016;30:93–101. [DOI] [PubMed] [Google Scholar]
- 14. Norris GH, Porter CM, Jiang C, et al. Dietary milk sphingomyelin reduces systemic inflammation in diet-induced obese mice and inhibits LPS activity in macrophages. Beverages. 2017;3:37. [Google Scholar]
- 15. Vincent GE, Jay SM, Sargent C, et al. Improving cardiometabolic health with diet, physical activity, and breaking up sitting: what about sleep? Front Physiol. 2017;8:865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Davidson DM. An introduction to cardiovascular disease. In: Shumaker SA, Czajkowski SM, eds. Social Support and Cardiovascular Disease. Boston, MA: Springer US; 1994: [Google Scholar]
- 17. Alberti KG, Eckel RH, Grundy SM, et al. ; International Association for the Study of Obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and Circulation. 2009;120:1640–1645. [DOI] [PubMed] [Google Scholar]
- 18. Lind L, Sundström J, Ärnlöv J, et al. A longitudinal study over 40 years to study the metabolic syndrome as a risk factor for cardiovascular diseases. Sci Rep. 2021;11:2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mensah GA, Roth GA, Fuster V.. The global burden of cardiovascular diseases and risk factors: 2020 and beyond. J Am Coll Cardiol. 2019;74:2529–2532. [DOI] [PubMed] [Google Scholar]
- 20. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: executive Summary: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74:1376–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017-2018. Data Brief no. 360. NCHS Data Brief, no 360. Hyattsville, MD: National Center for Health Statistics; 2020. [PubMed]
- 22. Hirode G, Wong RJ.. Trends in the prevalence of metabolic syndrome in the United States, 2011-2016. JAMA. 2020;323:2526–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Spengler EK, Loomba R.. Recommendations for diagnosis, referral for liver biopsy, and treatment of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Mayo Clin Proc. 2015;90:1233–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Younossi ZM, Tampi R, Priyadarshini M, et al. Burden of illness and economic model for patients with nonalcoholic steatohepatitis in the United States. Hepatology. 2019;69:564–572. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA: U.S. Dept of Health and Human Services; 2020.
- 26. Yusuf S, Hawken S, Ôunpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. [DOI] [PubMed] [Google Scholar]
- 27. Minihane AM, Vinoy S, Russell WR, et al. Low-grade inflammation, diet composition and health: current research evidence and its translation. Br J Nutr. 2015;114:999–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kopp W. How Western diet and lifestyle drive the pandemic of obesity and civilization diseases. Diabetes Metab Syndr Obes. 2019;12:2221–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shan Z, Rehm CD, Rogers G, et al. Trends in dietary carbohydrate, protein, and fat intake and diet quality among US adults, 1999-2016. JAMA. 2019;322:1178–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zilversmit DB. Atherogenesis: a postprandial phenomenon. Circulation. 1979;60:473–485. [DOI] [PubMed] [Google Scholar]
- 31. Rungratanawanich W, Qu Y, Wang X, et al. Advanced glycation end products (AGEs) and other adducts in aging-related diseases and alcohol-mediated tissue injury. Exp Mol Med. 2021;53:168–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lassenius MI, Pietilainen KH, Kaartinen K, et al. ; on behalf of the FinnDiane Study Group. Bacterial endotoxin activity in human serum is associated with dyslipidemia, insulin resistance, obesity, and chronic inflammation. Diabetes Care. 2011;34:1809–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.US Department of Agriculture and US Department of Health and Human Services. Dietary Guidelines for Americans, 2020-2025 9th ed. Washington, DC; 2020. Available at: DietaryGuidelines.gov.
- 34. Drouin-Chartier JP, Côté JA, Labonté M, et al. Comprehensive review of the impact of dairy foods and dairy fat on cardiometabolic risk. Adv Nutr. 2016;7:1041–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Le TT, Phan TTQ, Van Camp J, et al. Milk and dairy polar lipids: occurrence, purification, and nutritional and technological properties. In: Ahmad MU, Xu X, eds. Polar Lipids. Elsevier / AOCS Press (Urbana, IL); 2015. [Google Scholar]
- 36. Vanderghem C, Bodson P, Danthine S, et al. Milk fat globule membrane and buttermilks: from composition to valorization. Biotechnol Agron Soc Environ. 2010;14:485–500. [Google Scholar]
- 37. Greenwood SL, Honan MC.. Symposium review: characterization of the bovine milk protein profile using proteomic techniques. J Dairy Sci. 2019;102:2796–2806. [DOI] [PubMed] [Google Scholar]
- 38. Goulding DA, Fox PF, O’Mahony JA.. Milk proteins: an overview. In: Boland M, Singh H, eds. Milk Proteins. 3rd edn. Academic Press (London, UK); 2020. [Google Scholar]
- 39. Lee FA. Milk and milk products. In: Lee FA, ed. Basic Food Chemistry. Dordrecht: Springer Netherlands; 1983. [Google Scholar]
- 40. Mather IH. A review and proposed nomenclature for major proteins of the milk-fat globule membrane. J Dairy Sci. 2000;83:203–247. [DOI] [PubMed] [Google Scholar]
- 41. Fong BY, Norris CS, MacGibbon AKH.. Protein and lipid composition of bovine milk-fat-globule membrane. Int Dairy J. 2007;17:275–288. [Google Scholar]
- 42. Jensen RG. The composition of bovine milk lipids: January 1995 to December 2000. J Dairy Sci. 2002;85:295–350. [DOI] [PubMed] [Google Scholar]
- 43. Yener S, van Valenberg HJF.. Characterisation of triacylglycerols from bovine milk fat fractions with MALDI-TOF-MS fragmentation. Talanta. 2019;204:533–541. [DOI] [PubMed] [Google Scholar]
- 44. Soggiu A, Roncada P, Piras C.. Proteomics in milk and dairy products. In: De Almeida AM, Eckersall D, Miller I, eds. Proteomics in Domestic Animals: From Farm to Systems Biology. Cham: Springer International Publishing; 2018. [Google Scholar]
- 45. Herreid EO. The Babcock test; a review of the literature. J Dairy Sci. 1942;25:335–370. [Google Scholar]
- 46. Månsson HL. Fatty acids in bovine milk fat. Food Nutr Res. 2008;52. doi:10.3402/fnr.v52i0.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bainbridge ML, Egolf E, Barlow JW, et al. Milk from cows grazing on cool-season pastures provides an enhanced profile of bioactive fatty acids compared to those grazed on a monoculture of pearl millet. Food Chem. 2017;217:750–755. [DOI] [PubMed] [Google Scholar]
- 48. Hess JM, Cifelli CJ, Fulgoni VL III. Energy and nutrient intake of Americans according to meeting current dairy recommendations. Nutrients. 2020;12:3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tettamanti G. Ganglioside/glycosphingolipid turnover: new concepts. Glycoconj J. 2004;20:301–317. [DOI] [PubMed] [Google Scholar]
- 50. Contarini G, Povolo M.. Phospholipids in milk fat: composition, biological and technological significance, and analytical strategies. Int J Mol Sci. 2013;14:2808–2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fox PF, McSweeney PLH.. Advanced Dairy Chemistry. New York, NY: Springer; 2009. [Google Scholar]
- 52. Morrison WR, Hay JD.. Polar lipids in bovine milk II. Long-chain bases, normal and 2-hydroxy fatty acids, and isomeric cis and trans monoenoic fatty acids in the sphingolipids. Biochim Biophys Acta. 1970;202:460–467. [DOI] [PubMed] [Google Scholar]
- 53. Liu Z, Li C, Pryce J, et al. Comprehensive characterization of bovine milk lipids: phospholipids, sphingolipids, glycolipids, and ceramides. J Agric Food Chem. 2020;68:6726–6738. [DOI] [PubMed] [Google Scholar]
- 54. Gallier S, Gragson D, Cabral C, et al. Composition and fatty acid distribution of bovine milk phospholipids from processed milk products. J Agric Food Chem. 2010;58:10503–10511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rombaut R, Camp JV, Dewettinck K.. Phospho- and sphingolipid distribution during processing of milk, butter and whey. Int J Food Sci Techol. 2006;41:435–443. [Google Scholar]
- 56. López-García G, Cilla A, Barberá R, et al. Effect of a milk-based fruit beverage enriched with plant sterols and/or galactooligosaccharides in a murine chronic colitis model. Foods. 2019;8:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chen GC, Szeto IM, Chen LH, et al. Dairy products consumption and metabolic syndrome in adults: systematic review and meta-analysis of observational studies. Sci Rep. 2015;5:14606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Soedamah-Muthu SS, de Goede J.. Dairy consumption and cardiometabolic diseases: systematic review and updated meta-analyses of prospective cohort studies. Curr Nutr Rep. 2018;7:171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chen GC, Wang Y, Tong X, et al. Cheese consumption and risk of cardiovascular disease: a meta-analysis of prospective studies. Eur J Nutr. 2017;56:2565–2575. [DOI] [PubMed] [Google Scholar]
- 60. de Goede J, Soedamah‐Muthu SS, Pan A, et al. Dairy consumption and risk of stroke: a systematic review and updated dose-response meta-analysis of prospective cohort studies. J Am Heart Assoc. 2016;5:E002787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Guo J, Astrup A, Lovegrove JA, et al. Milk and dairy consumption and risk of cardiovascular diseases and all-cause mortality: dose-response meta-analysis of prospective cohort studies. Eur J Epidemiol. 2017;32:269–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dehghan M, Mente A, Rangarajan S, et al. ; Prospective Urban Rural Epidemiology (PURE) study investigators. Association of dairy intake with cardiovascular disease and mortality in 21 countries from five continents (PURE): a prospective cohort study. Lancet. 2018;392:2288–2297. [DOI] [PubMed] [Google Scholar]
- 63. Abreu S, Moreira P, Moreira C, et al. Intake of milk, but not total dairy, yogurt, or cheese, is negatively associated with the clustering of cardiometabolic risk factors in adolescents. Nutr Res. 2014;34:48–57. [DOI] [PubMed] [Google Scholar]
- 64. Beydoun MA, Fanelli-Kuczmarski MT, Beydoun HA, et al. Dairy product consumption and its association with metabolic disturbance in a prospective study of urban adults. Br J Nutr. 2018;119:706–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bhavadharini B, Dehghan M, Mente A, et al. Association of dairy consumption with metabolic syndrome, hypertension and diabetes in 147 812 individuals from 21 countries. BMJ Open Diabetes Res Care. 2020;8:E000826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Johansson I, Esberg A, Nilsson LM, et al. Dairy product intake and cardiometabolic diseases in northern Sweden: a 33-year prospective cohort study. Nutrients. 2019;11:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yu E, Hu FB.. Dairy products, dairy fatty acids, and the prevention of cardiometabolic disease: a review of recent evidence. Curr Atheroscler Rep. 2018;20:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Thorning TK, Bertram HC, Bonjour JP, et al. Whole dairy matrix or single nutrients in assessment of health effects: current evidence and knowledge gaps. Am J Clin Nutr. 2017;105:1033–1045. [DOI] [PubMed] [Google Scholar]
- 69. Trimigno A, Münger L, Picone G, et al. GC-MS based metabolomics and NMR spectroscopy investigation of food intake biomarkers for milk and cheese in serum of healthy humans. Metabolites. 2018;8:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Risérus U, Marklund M.. Milk fat biomarkers and cardiometabolic disease. Curr Opin Lipidol. 2017;28:46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yoo W, Gjuka D, Stevenson HL, et al. Fatty acids in non-alcoholic steatohepatitis: focus on pentadecanoic acid. PLoS One. 2017;12:E0189965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kratz M, Marcovina S, Nelson JE, et al. Dairy fat intake is associated with glucose tolerance, hepatic and systemic insulin sensitivity, and liver fat but not β-cell function in humans. Am J Clin Nutr. 2014;99:1385–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Imamura F, Fretts A, Marklund M, et al. ; InterAct Consortium. Fatty acid biomarkers of dairy fat consumption and incidence of type 2 diabetes: a pooled analysis of prospective cohort studies. PLoS Med. 2018;15:E1002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Louie JCY, Flood VM, Hector DJ, et al. Dairy consumption and overweight and obesity: a systematic review of prospective cohort studies. Obes Rev. 2011;12:e582–e592. [DOI] [PubMed] [Google Scholar]
- 75. Kratz M, Baars T, Guyenet S.. The relationship between high-fat dairy consumption and obesity, cardiovascular, and metabolic disease. Eur J Nutr. 2013;52:1–24. [DOI] [PubMed] [Google Scholar]
- 76. Chen M, Pan A, Malik VS, et al. Effects of dairy intake on body weight and fat: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012;96:735–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wang H, Troy LM, Rogers GT, et al. Longitudinal association between dairy consumption and changes of body weight and waist circumference: The Framingham Heart Study. Int J Obes. 2014;38:299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Schwingshackl L, Hoffmann G, Schwedhelm C, et al. Consumption of dairy products in relation to changes in anthropometric variables in adult populations: a systematic review and meta-analysis of cohort studies. PLoS One. 2016;11:E0157461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lu L, Xun P, Wan Y, et al. Long-term association between dairy consumption and risk of childhood obesity: a systematic review and meta-analysis of prospective cohort studies. Eur J Clin Nutr. 2016;70:414–423. [DOI] [PubMed] [Google Scholar]
- 80. O'Sullivan TA, Schmidt KA, Kratz M.. Whole-fat or reduced-fat dairy product intake, adiposity, and cardiometabolic health in children: a systematic review. Adv Nutr. 2020;11:928–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Dror D. Dairy consumption and pre‐school, school‐age and adolescent obesity in developed countries: a systematic review and meta‐analysis. Obes Rev. 2014;15:516–527. [DOI] [PubMed] [Google Scholar]
- 82. Beck AL, Heyman M, Chao C, et al. Full fat milk consumption protects against severe childhood obesity in Latinos. Prev Med Rep. 2017;8:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Dougkas A, Barr S, Reddy S, et al. A critical review of the role of milk and other dairy products in the development of obesity in children and adolescents. Nutr Res Rev. 2019;32:106–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Schakel SF. Maintaining a nutrient database in a changing marketplace: keeping pace with changing food products—a research perspective. J Food Compos Anal. 2001;14:315–322. [Google Scholar]
- 85. Menotti A, Keys A, Aravanis C, et al. Seven countries study. First 20-year mortality data in 12 cohorts of six countries. Ann Med. 1989;21:175–179. [DOI] [PubMed] [Google Scholar]
- 86. Kromhout D, Bloemberg B, Feskens E, et al. Saturated fat, vitamin C and smoking predict long-term population all-cause mortality rates in the Seven Countries Study. Int J Epidemiol. 2000;29:260–265. [DOI] [PubMed] [Google Scholar]
- 87. Jacobson TA, Ito MK, Maki KC, et al. National lipid association recommendations for patient-centered management of dyslipidemia: part 1–full report. J Clin Lipidol. 2015;9:129–169. [DOI] [PubMed] [Google Scholar]
- 88. Americal College of Cardiology; Americal Heart Association. Reprint: 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk. J Am Pharm Assoc (2003) 2014;54:E2. [DOI] [PubMed] [Google Scholar]
- 89. Poppitt SD. Cow's milk and dairy consumption: is there now consensus for cardiometabolic health? Front Nutr. 2020;7:574725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. de Souza RJ, Mente A, Maroleanu A, et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ. 2015;351:h3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Astrup A, Magkos F, Bier DM, et al. Saturated fats and health: a reassessment and proposal for food-based recommendations: JACC state-of-the-art review. J Am Coll Cardiol. 2020;76:844–857. [DOI] [PubMed] [Google Scholar]
- 92. Sjogren P, Rosell M, Skoglund-Andersson C, et al. Milk-derived fatty acids are associated with a more favorable LDL particle size distribution in healthy men. J Nutr. 2004;134:1729–1735. [DOI] [PubMed] [Google Scholar]
- 93. Volk BM, Kunces LJ, Freidenreich DJ, et al. Effects of step-wise increases in dietary carbohydrate on circulating saturated fatty acids and palmitoleic acid in adults with metabolic syndrome. PLoS One. 2014;9:E113605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hodge AM, English DR, O'Dea K, et al. Plasma phospholipid and dietary fatty acids as predictors of type 2 diabetes: interpreting the role of linoleic acid. Am J Clin Nutr. 2007;86:189–197. [DOI] [PubMed] [Google Scholar]
- 95. Patel PS, Sharp SJ, Jansen E, et al. Fatty acids measured in plasma and erythrocyte-membrane phospholipids and derived by food-frequency questionnaire and the risk of new-onset type 2 diabetes: a pilot study in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohort. Am J Clin Nutr. 2010;92:1214–1222. [DOI] [PubMed] [Google Scholar]
- 96. Vessby B, Aro A, Skarfors E, et al. The risk to develop NIDDM is related to the fatty acid composition of the serum cholesterol esters. Diabetes. 1994;43:1353–1357. [DOI] [PubMed] [Google Scholar]
- 97. Wang L, Folsom AR, Zheng ZJ, ARIC Study Investigators, et al. Plasma fatty acid composition and incidence of diabetes in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr. 2003;78:91–98. [DOI] [PubMed] [Google Scholar]
- 98. Warensjö E, Risérus U, Vessby B.. Fatty acid composition of serum lipids predicts the development of the metabolic syndrome in men. Diabetologia. 2005;48:1999–2005. [DOI] [PubMed] [Google Scholar]
- 99. Zong G, Zhu J, Sun L, et al. Associations of erythrocyte fatty acids in the de novo lipogenesis pathway with risk of metabolic syndrome in a cohort study of middle-aged and older Chinese. Am J Clin Nutr. 2013;98:319–326. [DOI] [PubMed] [Google Scholar]
- 100. Maki KC, Slavin JL, Rains TM, et al. Limitations of observational evidence: implications for evidence-based dietary recommendations. Adv Nutr. 2014;5:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Anto L, Warykas SW, Torres-Gonzalez M, et al. Milk polar lipids: underappreciated lipids with emerging health benefits. Nutrients. 2020;12:1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Christie WW, Noble RC, Davies G.. Phospholipids in milk and dairy products. Int J Dairy Technol. 1987;40:10–12. [Google Scholar]
- 103. Ohlsson L, Burling H, Duan RD, et al. Effects of a sphingolipid-enriched dairy formulation on postprandial lipid concentrations. Eur J Clin Nutr. 2010;64:1344–1349. [DOI] [PubMed] [Google Scholar]
- 104. Keller S, Malarski A, Reuther C, et al. Milk phospholipid and plant sterol-dependent modulation of plasma lipids in healthy volunteers. Eur J Nutr. 2013;52:1169–1179. [DOI] [PubMed] [Google Scholar]
- 105. Ramprasath VR, Jones PJ, Buckley DD, et al. Effect of dietary sphingomyelin on absorption and fractional synthetic rate of cholesterol and serum lipid profile in humans. Lipids Health Dis. 2013;12:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Weiland A, Bub A, Barth SW, et al. Effects of dietary milk- and soya-phospholipids on lipid-parameters and other risk indicators for cardiovascular diseases in overweight or obese men - two double-blind, randomised, controlled, clinical trials. J Nutr Sci. 2016;5:E21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Rosqvist F, Smedman A, Lindmark-Månsson H, et al. Potential role of milk fat globule membrane in modulating plasma lipoproteins, gene expression, and cholesterol metabolism in humans: a randomized study. Am J Clin Nutr. 2015;102:20–30. [DOI] [PubMed] [Google Scholar]
- 108. Beals E, Kamita SG, Sacchi R, et al. Addition of milk fat globule membrane-enriched supplement to a high-fat meal attenuates insulin secretion and induction of soluble epoxide hydrolase gene expression in the postprandial state in overweight and obese subjects. J Nutr Sci. 2019;8:E16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Demmer E, Van Loan MD, Rivera N, et al. Addition of a dairy fraction rich in milk fat globule membrane to a high-saturated fat meal reduces the postprandial insulinaemic and inflammatory response in overweight and obese adults. J Nutr Sci. 2016;5:E14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Conway V, Couture P, Richard C, et al. Impact of buttermilk consumption on plasma lipids and surrogate markers of cholesterol homeostasis in men and women. Nutr Metab Cardiovasc Dis. 2013;23:1255–1262. [DOI] [PubMed] [Google Scholar]
- 111. Conway V, Couture P, Gauthier S, et al. Effect of buttermilk consumption on blood pressure in moderately hypercholesterolemic men and women. Nutrition. 2014;30:116–119. [DOI] [PubMed] [Google Scholar]
- 112. Ten Bruggencate SJ, Frederiksen PD, Pedersen SM, et al. Dietary milk-fat-globule membrane affects resistance to diarrheagenic Escherichia coli in healthy adults in a randomized, placebo-controlled, double-blind study. J Nutr. 2016;146:249–255. [DOI] [PubMed] [Google Scholar]
- 113. Ohlsson L, Hertervig E, Jönsson BAG, et al. Sphingolipids in human ileostomy content after meals containing milk sphingomyelin. Am J Clin Nutr. 2010;91:672–678. [DOI] [PubMed] [Google Scholar]
- 114. Millar CL, Jiang C, Norris GH, et al. Cow's milk polar lipids reduce atherogenic lipoprotein cholesterol, modulate gut microbiota and attenuate atherosclerosis development in LDL-receptor knockout mice fed a Western-type diet. J Nutr Biochem. 2020;79:108351. [DOI] [PubMed] [Google Scholar]
- 115. Milard M, Laugerette F, Durand A, et al. Milk polar lipids in a high-fat diet can prevent body weight gain: modulated abundance of gut bacteria in relation with fecal loss of specific fatty acids. Mol Nutr Food Res. 2019;63:1801078. [DOI] [PubMed] [Google Scholar]
- 116. Milard M, Penhoat A, Durand A, et al. Acute effects of milk polar lipids on intestinal tight junction expression: towards an impact of sphingomyelin through the regulation of IL-8 secretion? J Nutr Biochem. 2019;65:128–138. [DOI] [PubMed] [Google Scholar]
- 117. Norris GH, Porter CM, Jiang C, et al. Dietary sphingomyelin attenuates hepatic steatosis and adipose tissue inflammation in high-fat-diet-induced obese mice. J Nutr Biochem. 2017;40:36–43. [DOI] [PubMed] [Google Scholar]
- 118. Lecomte M, Couëdelo L, Meugnier E, et al. Dietary emulsifiers from milk and soybean differently impact adiposity and inflammation in association with modulation of colonic goblet cells in high-fat fed mice. Mol Nutr Food Res. 2016;60:609–620. [DOI] [PubMed] [Google Scholar]
- 119. Wat E, Tandy S, Kapera E, et al. Dietary phospholipid-rich dairy milk extract reduces hepatomegaly, hepatic steatosis and hyperlipidemia in mice fed a high-fat diet. Atherosclerosis. 2009;205:144–150. [DOI] [PubMed] [Google Scholar]
- 120. Kamili A, Wat E, Chung RWS, et al. Hepatic accumulation of intestinal cholesterol is decreased and fecal cholesterol excretion is increased in mice fed a high-fat diet supplemented with milk phospholipids. Nutr Metab. 2010;7:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Mathiassen JH, Nejrup RG, Frøkiaer H, et al. Emulsifying triglycerides with dairy phospholipids instead of soy lecithin modulates gut lipase activity. Eur J Lipid Sci Technol. 2015;117:1522–1539. [Google Scholar]
- 122. Lecomte M, Bourlieu C, Meugnier E, et al. Milk polar lipids affect in vitro digestive lipolysis and postprandial lipid metabolism in mice. J Nutr. 2015;145:1770–1777. [DOI] [PubMed] [Google Scholar]
- 123. Eckhardt ER, Wang DQH, Donovan JM, et al. Dietary sphingomyelin suppresses intestinal cholesterol absorption by decreasing thermodynamic activity of cholesterol monomers. Gastroenterology. 2002;122:948–956. [DOI] [PubMed] [Google Scholar]
- 124. Li T, Du M, Wang H, et al. Milk fat globule membrane and its component phosphatidylcholine induce adipose browning both in vivo and in vitro. J Nutr Biochem. 2020;81:108372. [DOI] [PubMed] [Google Scholar]
- 125. Heid HW, Keenan TW.. Intracellular origin and secretion of milk fat globules. Eur J Cell Biol. 2005;84:245–258. [DOI] [PubMed] [Google Scholar]
- 126. Timby N, Domellöf M, Lönnerdal B, et al. Supplementation of infant formula with bovine milk fat globule membranes. Adv Nutr. 2017;8:351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Noh SK, Koo SI.. Milk sphingomyelin is more effective than egg sphingomyelin in inhibiting intestinal absorption of cholesterol and fat in rats. J Nutr. 2004;134:2611–2616. [DOI] [PubMed] [Google Scholar]
- 128. Koo SI, Noh SK.. Phosphatidylcholine inhibits and lysophosphatidylcholine enhances the lymphatic absorption of α-tocopherol in adult rats. J Nutr. 2001;131:717–722. [DOI] [PubMed] [Google Scholar]
- 129. Burling H, Graverholt G.. Milk – a new source for bioactive phospholipids for use in food formulations. Lipid Technol. 2008;20:229–231. [Google Scholar]
- 130. Kuikka M, Ramstedt B, Ohvo-Rekilä H, et al. Membrane properties of d-erythro-N-acyl sphingomyelins and their corresponding dihydro species. Biophys J. 2001;80:2327–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Cohn JS, Wat E, Kamili A, et al. Dietary phospholipids, hepatic lipid metabolism and cardiovascular disease. Curr Opin Lipidol. 2008;19:257–262. [DOI] [PubMed] [Google Scholar]
- 132. Aziz MM, Ishihara S, Mishima Y, et al. MFG-E8 attenuates intestinal inflammation in murine experimental colitis by modulating osteopontin-dependent alphavbeta3 integrin signaling. J Immunol. 2009;182:7222–7232. [DOI] [PubMed] [Google Scholar]
- 133. Bu HF, Zuo XL, Wang X, et al. Milk fat globule-EGF factor 8/lactadherin plays a crucial role in maintenance and repair of murine intestinal epithelium. J Clin Invest. 2007;117:3673–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Conway V, Gauthier SF, Pouliot Y.. Effect of cream pasteurization, microfiltration and enzymatic proteolysis on in vitro cholesterol-lowering activity of buttermilk solids. Dairy Sci Technol. 2010;90:16–35.. [Google Scholar]
- 135. Yamazaki Y, Kondo K, Maeba R, et al. Proportion of nervonic acid in serum lipids is associated with serum plasmalogen levels and metabolic syndrome. J Oleo Sci. 2014;63:16–35.. [DOI] [PubMed] [Google Scholar]
- 136. Kirby RJ, Zheng S, Tso P, et al. Bile salt-stimulated carboxyl ester lipase influences lipoprotein assembly and secretion in intestine: a process mediated via ceramide hydrolysis. J Biol Chem. 2002;277:16–35.. [DOI] [PubMed] [Google Scholar]
- 137. Martins IJ, Mortimer BC, Miller J, et al. Effects of particle size and number on the plasma clearance of chylomicrons and remnants. J Lipid Res. 1996;37:16–35.. [PubMed] [Google Scholar]
- 138. Imaizumi K, Tominaga A, Sato M, et al. Effects of dietary sphingolipids on levels of serum and liver lipids in rats. Nutr Res. 1992;12:16–35.. [Google Scholar]
- 139. Ohlsson L, Burling H, Nilsson A.. Long term effects on human plasma lipoproteins of a formulation enriched in butter milk polar lipid. Lipids Health Dis. 2009;8:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Mah E, Bruno RS.. Postprandial hyperglycemia on vascular endothelial function: mechanisms and consequences. Nutr Res. 2012;32:16–35.. [DOI] [PubMed] [Google Scholar]
- 141. Jacome-Sosa M, Parks EJ, Bruno RS, et al. Postprandial metabolism of macronutrients and cardiometabolic risk: recent developments, emerging concepts, and future directions. Adv Nutr. 2016;7:16–35.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Traber MG, Buettner GR, Bruno RS.. The relationship between vitamin C status, the gut-liver axis, and metabolic syndrome. Redox Biol. 2019;21:101091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:16–35.. [DOI] [PubMed] [Google Scholar]
- 144. Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:16–35.. [DOI] [PubMed] [Google Scholar]
- 145. Yang Y, Zhang T, Zhou G, et al. Prevention of necrotizing enterocolitis through milk polar lipids reducing intestinal epithelial apoptosis. J Agric Food Chem. 2020;68:16–35.. [DOI] [PubMed] [Google Scholar]
- 146. Snow DR, Ward RE, Olsen A, et al. Membrane-rich milk fat diet provides protection against gastrointestinal leakiness in mice treated with lipopolysaccharide. J Dairy Sci. 2011;94:16–35.. [DOI] [PubMed] [Google Scholar]
- 147. Palmano KP, MacGibbon AKH, Gunn CA, et al. In vitro and in vivo anti-inflammatory activity of bovine milkfat globule (MFGM)-derived complex lipid fractions. Nutrients. 2020;12:2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Ferraris RP, Vinnakota RR.. Intestinal nutrient transport in genetically obese mice. Am J Clin Nutr. 1995;62:16–35.. [DOI] [PubMed] [Google Scholar]
- 149. Brun P, Castagliuolo I, Di Leo V, et al. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G518–25.. [DOI] [PubMed] [Google Scholar]
- 150. Reis MG, Roy NC, Bermingham EN, . et al. Impact of dietary dairy polar lipids on lipid metabolism of mice fed a high-fat diet. J Agric Food Chem. 2013;61:2729–2738. 10.1021/jf303795b 23394615 [DOI] [PubMed] [Google Scholar]
- 151. Le Barz M, Vors C, Combe E, et al. Milk polar lipids favorably alter circulating and intestinal ceramide and sphingomyelin species in postmenopausal women. JCI Insight. 2021;6:146161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Rocha-Mendoza D, Kosmerl E, Miyagusuku-Cruzado G, et al. Growth of lactic acid bacteria in milk phospholipids enhances their adhesion to Caco-2 cells. J Dairy Sci. 2020;103:16–35.. [DOI] [PubMed] [Google Scholar]
- 153. Galley JD, Yu Z, Kumar P, et al. The structures of the colonic mucosa-associated and luminal microbial communities are distinct and differentially affected by a prolonged murine stressor. Gut Microbes. 2014;5:16–35.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.US Department of Agriculture Economic Research Service. Dairy Data. 2021. Available at: https://www.ers.usda.gov/data-products/dairy-data/. Accessed August 19, 2021.
- 155. Bourlieu C, Michalski M-C.. Structure–function relationship of the milk fat globule. Curr Opin Clin Nutr Metab Care. 2015;18(2):118-127. [DOI] [PubMed] [Google Scholar]
- 156.National Institutes of Health. 2020-2030 Strategic Plan for NIH Nutrition Research. A Report of the NIH Nutrition Research Task Force. 2020. Available at: https://www.niddk.nih.gov/about-niddk/strategic-plans-reports/strategic-plan-nih-nutrition-research. Accessed August 19, 2021.