Abstract
Addition of pre- and probiotics may confer growth and health benefits when added to the diet of pigs. To determine the effects of feeding mannan oligosaccharide (MOS) and Lactobacillus mucosae (LM) as prebiotic and probiotic sources in weanling pigs under immune challenge, 96 weaned pigs were randomly allotted to 16 experimental pens within a 2 × 2 factorial arrangement of treatments. Control diets with or without 0.1% yeast-derived MOS were randomly assigned to pens and 109 cfu/pig LM broth or a control broth were top-dressed daily. Pigs were fed one of four dietary treatments (control, MOS, LM, and MOS+LM) in Phases I and II (days 0 to 7 and days 7 to 21 postweaning, respectively) and a common diet during Phase III (days 21 to 35 postweaning). On day 14, all pigs were challenged with 100 µg/kg body weight (BW) Escherichia coli lipopolysaccharide (LPS) via intraperitonial injection. Feed disappearance and pig BW were measured weekly. Blood and fecal samples were collected weekly, and additional blood samples were collected on days 1 and 3 post-LPS challenge. On days 15 and 21, one pig per pen was euthanized for collection of ileal mucosa and duodenal and ileal tissue samples. From days 0 to 14, feeding LM decreased gain-to-feed ratio (G:F; P < 0.05). An interaction between LM and MOS was observed for G:F on days 14 to 21 (P < 0.05); G:F in LM (715 g/kg) was greater compared with MOS+LM (P < 0.05; 600 g/kg) and control (P < 0.10; 615 g/kg), but was not different (P > 0.10) from MOS (674 g/kg). After pigs were fed a common diet (days 21 to 35), G:F was decreased (P < 0.05) in the LM treatment groups. Pigs fed diets that included MOS had increased serum immunoglobulin (Ig) G on days 1 and 3 post-LPS challenge and 2 wk after removal of treatments (P < 0.05) and on days 14 and 21 postweaning (P < 0.10) compared with pigs fed diets without MOS. On day 15, mucosal immunoglobulin G was increased (P < 0.05) in control vs. MOS and LM groups. Circulating IL-1β in control and MOS+LM pigs increased (P < 0.05) on day 1 post-LPS challenge but did not change (P > 0.10) in MOS and LM groups. On day 15, pigs fed LM had decreased (P < 0.05) ileal crypt depth compared with pigs fed the control diet. On day 21, fecal propionate and butyrate tended to be lower (P < 0.10) in pigs fed MOS vs. control and MOS+LM diet. These preliminary findings suggest that feeding LM alone improved feed efficiency and ileal morphological structure during the first week of LPS challenge; additionally, feeding LM and MOS may have beneficial effects relative to immune biomarkers.
Keywords: growth, gut health, immunity, pigs, prebiotic, probiotic
Introduction
Numerous studies in pigs have been conducted using prebiotics as antibiotic alternatives. Fermentable ingredients, such as resistant starch, nonstarch polysaccharides, unabsorbed sugars, and oligosaccharides have been investigated (Zhao et al., 2013; Sun et al., 2015; McDonnell et al., 2016). Among these, mannan oligosaccharides (MOS) have been shown to improve growth performance (Zhou et al., 2020) and nutrient digestibility in weanling pigs (Zhao et al., 2012). Recent studies indicate that MOS may increase butyrate-producing bacteria (Zhou et al., 2020) and modulate gut inflammatory response and villus height (VH; Agazzi et al., 2020) of weanling pigs, suggesting a potential prebiotic effect of MOS. We identified a potential probiotic species, Lactobacillus mucosae (LM), that was increased by feeding MOS and was positively correlated with circulating immunoglobulin (Ig) A concentration (data not published). Releasing of immunoglobulin A (IgA) antibodies is associated with mucosal immunity (Burkey et al., 2009). An in vitro study showed that LM had the best adhesive capacity among all the tested Lactobacillus species; LM also revealed inhibitory effects on pathogenic Escherichia coli and Salmonella species (Valeriano et al., 2014). Therefore, our objective was to investigate the effects of MOS, LM, and the combination of MOS and LM, on growth performance, immune response, and gut health of weanling pigs challenged with E. coli lipopolysaccharide (LPS) during the nursery period.
Materials and Methods
The experimental protocol was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Nebraska–Lincoln with IACUC #1319 and adhered to the Guide for the Care and Use of Agricultural Animals in Research and Teaching (FASS, 2010).
Animals
A total of 100 pigs (including four extra pigs; Large white × Landrace × Yorkshire) were selected from the Eastern Nebraska Research and Extension Center Swine Unit (Mead, NE) and transported to the University of Nebraska–Lincoln campus. Pigs were weaned on day 23 (±0.5 d) postfarrowing (average initial body weight [BW] = 5.90 ± 0.18 kg) and were randomly allotted to 16 experimental pens with a 2 × 2 factorial arrangement of treatments (four pens per treatment; with six pigs per pen; three males and three females per pen). The four extra pigs were euthanized immediately after weaning for collection of baseline mucosal and tissue samples. Pigs were given ad libitum access to feed and water. Heat lamps were used to maintain room temperature at 27 °C during the first 2 wk and the temperature were gradually decreased (1 °C/wk) thereafter. On day 14 postweaning, all pigs were challenged with 100 µg/kg BW of E. coli LPS (E. coli serotype O55:B5, Sigma Chemical, St. Louis, MO) via intraperitoneal injection (Liu et al., 2012).
Diets
Corn-soybean-meal-based diets (Table 1; without plasma or antibiotics), formulated to meet or exceed NRC (2012) recommendations, with or without 0.1% yeast-derived MOS were randomly assigned to pens and 109 cfu/pig LM broth or an equal volume of control broth (20% glycerol in peptone water) was top-dressed daily. Pigs were fed one of the four dietary treatments (control, MOS, LM, and MOS+LM) in Phase I and Phase II (days 0 to 7 and days 7 to 21 postweaning, respectively). A common diet was fed to all pigs during Phase III (days 21 to 35 postweaning) to investigate the carryover effects of dietary treatments.
Table 1.
Ingredient and chemical composition of the control diets (as-fed basis)
| Item | Phase I1 | Phase II1 | Phase III |
|---|---|---|---|
| (days 0 to 7) | (days 7 to 21) | (days 21 to 35) | |
| Ingredients, % | |||
| Corn | 53.06 | 53.46 | 55.80 |
| Soybean meal, 47.7% CP | 15.00 | 20.00 | 35.00 |
| Whey powder | 10.00 | 10.00 | 0.00 |
| Fish meal | 8.00 | 8.00 | 6.00 |
| Soy protein concentrate | 10.00 | 5.00 | 0.00 |
| Corn oil | 1.50 | 1.50 | 1.50 |
| Starch | 0.10 | 0.10 | 0.00 |
| Dicalcium phosphate, 18.5% P | 0.40 | 0.26 | 0.28 |
| Limestone | 0.74 | 0.70 | 0.72 |
| Salt | 0.30 | 0.30 | 0.30 |
| Vitamin premix2 | 0.25 | 0.25 | 0.25 |
| Trace mineral premix3 | 0.15 | 0.15 | 0.15 |
| l-Lys-HCL | 0.30 | 0.20 | 0.00 |
| dl-Met | 0.06 | 0.04 | 0.00 |
| l-Thr | 0.10 | 0.04 | 0.00 |
| l-Trp | 0.04 | 0.00 | 0.00 |
| Calculated composition4 | |||
| ME, kcal/kg | 3,452.00 | 3,429.00 | 3,388.00 |
| CP, % | 23.95 | 22.67 | 21.23 |
| STTD P, % | 0.45 | 0.43 | 0.36 |
| Total P, % | 0.71 | 0.67 | 0.62 |
| Ca, % | 0.86 | 0.81 | 0.71 |
| SID AA, % | |||
| Lys | 1.50 | 1.37 | 1.26 |
| Met | 0.44 | 0.42 | 0.38 |
| Met+Cys | 0.74 | 0.71 | 0.70 |
| Thr | 0.90 | 0.81 | 0.80 |
| Trp | 0.28 | 0.24 | 0.27 |
1In Phase I (days 1 to 14) and Phase II (days 15 to 28), 0.1% of mannan oligosaccharides (MOS; Saccharomyces cerevisiae; bioSecure, Princeton, MN) partially replaced the starch from the control diet for MOS and MOS+Lactobacillus mucosae (LM) treatment groups. A daily top dressing of 6 mL LM broth were given to LM and MOS+LM-treated pens to achieve approximately 109 cfu of LM per pig, while an equal volume of 20% glycerol in peptone water were given to control and MOS-treated pens. The volume of bacterial or control broth were adjusted according to pig number in each pen.
2Vitamin premix supplied, per kilogram of diet, 5,500 IU vitamin A (as retinyl acetate), 550 IU vitamin D (as cholecalciferol), 30 IU vitamin E (as tocopheryl acetate), 4.4 mg vitamin K (as menadione dimethylpyrimidinol bisulfate), 11.0 mg riboflavin, 22.05 mg D-pantothenic acid, 33.0 mg niacin, and 33.0 mg vitamin B12 (as cyanocobalamin).
3Trace mineral premix contained 10 mg/kg copper (as CuSO4∙5H2O), 0.25 mg/kg iodine (as Ca(IO3)∙H2O), 125 mg/kg iron (as FeSO4FeSO4∙2H2O), 15 mg/kg manganese (MnO), 0.3 mg/kg selenium (Na2SeO3), and 125 mg/kg zinc (ZnSO4∙H2O).
4ME = metabolizable energy; CP = crude protein; STTD = standardized total tract digestible; SID = standardized ileal digestible.
Preparation of L. mucosae broth
The LM primer was designed using the PrimerQuest program (https://www.idtdna.com/Primerquest) based on the genomic sequence of L. mucosae strain LM1 (Lee et al., 2012b), and the specificity was tested using Basic Local Alignment Search Tool (BLAST) under NCBI (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Two candidate primer sets were obtained from Integrated DNA Technologies (Coralville, IA) and tested using polymerase chain reaction (PCR) with fecal DNA samples that were previously evaluated for the presence or absence of LM. The positive PCR products were verified by DNA sequencing (Eurofins MWG Operon, Louisville, KY), and one primer set specific for LM was selected as follows: forward primer: 5′-CCGCATAACAATTTGAATCGCA-3′, reverse primer: 5′-GACTTTCTGGTTAGATACCGTCAC-3′ with an amplicon length of 338 base pair.
Fresh fecal samples collected from 10 nursery pigs (around 35 d of age and fed with a commercial nursery diet) were pooled and incubated anaerobically in an M9 Minimal Salts medium (0.5 g fecal/5 mL medium) at 37 °C overnight. Subsequently, a 10-fold dilution series of fecal culture in phosphate-buffered saline (PBS) was prepared. From each dilution sample, 50 µL was inoculated on a Lactobacilli De Man, Rogosa and Sharpe (MRS) agar plate (Difco Laboratories, Detroit, MI) and incubated for 60 to 72 h at 37 °C under anaerobic conditions. Random colonies were picked from the agar plates and incubated anaerobically at 37 °C for 60 to 72 h in 96-well culture plates containing 100 µL of MRS broth in each well. The bacterial broth (1 µL) from each well was directly transferred into a 96-well PCR plate with a reaction mixture (24 µL) of 12.5 µL 2× Terra PCR Direct Buffer (with Mg2+, dNTP), 15 pmol forward and reverse LM primers, respectively, 0.5 µL Terra PCR Direct Polymerase Mix, and 10-µL sterile water. The amplification program was 98 °C for 3 min, followed by 35 cycles of 98 °C for 10 s, 60 °C for 15 s, and 48 °C for 40 s. Amplification products were detected by agarose gel electrophoresis (5 µL PCR product, 1.5% agarose gel). Three positive PCR products were sequenced (Eurofins MWG Operon, Louisville, KY) to align with the genomic sequence of L. mucosae strain (Lee et al., 2012b) and one of them was selected as the LM source with a similarity of 98%. The original bacterial colonies (100 µL) in 96-well plates were mixed with 50 µL of 50% glycerol in Dunham’s peptone water and stored at −80 °C.
Before the animal experiment was initiated, the selected strain was cultured in 10 mL of MRS broth at 37 °C overnight and then transferred to 1 L of MRS broth and incubated at 37 °C while shaking at 225 rpm overnight (Oueue Orbital Shaker, Parkersburg, WV). A subsample of 250-µL LM broth in triplicate was measured for bacterial concentrations using a UV plate reader at 600 nm. The population of LM was calculated assuming there was 0.8 × 109 cfu/mL of broth for optical density (OD) = 1. The LM broth was centrifuged at 1,500 × g for 5 min at room temperature. The supernatant was removed, and the bacterial pellet was washed with suspension buffer (20% glycerol in Dunham’s peptone water). The bacterial pellet was resuspended in 20% glycerol to equate to approximately 1 × 109 cfu/mL of LM broth. Final concentration was 0.92 × 109 cfu/mL for the first batch, and 0.65 × 109 cfu/mL for the second batch.
The LM broth was aliquoted into sterile conical tubes to provide 1 × 109 cfu/pig for individual pens and stored in −80 °C before use. The 20% glycerol in Dunham’s peptone water was aliquoted at the same volume for each pen as a control broth. In the morning (0900) from days 1 to 20 postweaning, the thawed LM broth and control broth were diluted in 50 mL of distilled water and evenly poured into individual feed troughs according to the treatment arrangements.
Sample collection and processing
Individual BW and feed disappearance were measured weekly from days 0 to 35 postweaning for the determination of average daily gain (ADG), average daily feed intake (ADFI), and gain-to-feed ratio (G:F). Feeder weights were measured daily during the first week of post-LPS challenge for monitoring daily feed intake. Body surface temperature before LPS challenge and three continuous day postchallenge were measured at least three times at both ear bases using an infrared thermometer (Soerensen and Pedersen, 2015). The greatest thermometer reading for individual pigs was recorded. Weekly blood samples (3 to 5 mL; days 0 to 35 postweaning) were taken at the jugular vein from all pigs using glass blood tubes without anticoagulant. Additional blood samples were collected on days 1 and 3 post-LPS challenge (days 15 and 17 postweaning). Blood samples were centrifuged at 1,000 × g for 20 min at 4 °C for collection of serum and stored at −20 °C for immunoglobulin and cytokine analysis. From days 0 to 21, serum samples from four pigs per pen were used for immune analysis, while serum samples from two pigs per pen on day 35 were analyzed to investigate carryover effects. Fecal samples were collected from 1 pig per pen on days 0, 14, and 21 via rectal massage using fecal loops for analysis of volatile fatty acids (VFA).
The four extra pigs on days 0 and 1 pig (median BW) per pen on days 15 and 21 were euthanized by injecting pentobarbital sodium solution (Fatal-Plus, Vortech Pharmaceutical, Dearborn, MI) in the neck region via the jugular vein according to the manufacturer’s instruction. Following euthanasia, duodenal and ileal tissue samples and ileal mucosa were collected. The sections of the small intestine were delineated as: duodenum ducts, the pancreas, and the liver (gall bladder) and is up to 12 inches in length from the base of the stomach pyloric end; ileum is up to about 4 feet from the cecum (ileocecal valve). Samples from similar locations were collected for individual animals. Approximately 2-cm segments of duodenal and ileal tissue were rinsed with ice-cold PBS to remove the digesta and clean the tissues. The tissues were fixed in 4% chilled paraformaldehyde for 24 h and stored in 70% ethanol at 4 °C for histological analysis. Additionally, a 40-cm segment from ileum was collected and cut into several 10-cm segments, which was opened longitudinally and cleaned with ice-cold PBS for mucosal immunoglobulins and cytokine concentration analysis. Ileal mucosa was collected by scraping the mucosal surface with an autoclaved glass slide. Mucosal samples were frozen in liquid nitrogen immediately and stored at −80 °C thereafter. Chilled PBS was added to the mucosal samples at a ratio of 1 g of tissue/3 mL, followed by homogenation at 5,000 to 10,000 rpm for 2 min using a Polytron homogenizer (Brinkman PT 3000, Littau, Switzerland). The mucosal homogenates were centrifuged at 10,000 × g for 10 min at 4 °C, and the supernatants were collected and stored at −80 °C for analysis of immunoglobulins and cytokines. The protein concentrations of mucosal homogenates were measured using the A280 program of a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE).
Serum and mucosal immune measures
Circulating and secretory IgA, IgM, and IgG were quantified from serum and mucosal samples using porcine-specific enzyme linked immunosorbent assay (ELISA) kits (Bethyl Laboratories, Inc., Montgometry, TX). Serum was diluted at 1:1,000, 1:10,000, and 1:50,000 with assay buffer for analysis of IgA, IgM, and IgG, respectively, while mucosa samples were diluted at 1:200 for IgA and at 1:500 for IgM and IgG, respectively. The range of detection for IgA and IgM assays was 15.6 to 1,000 ng/mL, and 7.8 to 500 ng/mL for IgG assay. The intra-assay CV for serum and mucosal IgA, IgM, and IgG assays was 2.6% and 4.1%, 3.5% and 2.0%, and 2.8% and 2.9%, respectively. The interassay CV for IgA, IgM, and IgG (serum and mucosal assays were combined) was 2.9%, 4.5%, and 4.1%, respectively.
Serum and mucosal IL-1β concentrations were determined using a porcine duoset ELISA kit (R&D Systems, Minneapolis, MN) following the manufacturer’s procedure. The detection range was 62.5 to 4,000 pg/mL. A total of 73 of 384 (19%) serum samples were out of the detection range and considered 0 pg/mL. For mucosa samples, 1 of 16 samples on day 15 were not detectable and considered 0 pg/mL, while 10 of 16 samples on day 21 were not detectable. The intra-assay CV for serum and mucosal IL-1β was 4.5% and 5.3%, respectively. The interassay CV combining serum and mucosal IL-1β assays was 4.8%.
Gut histological measurements
The gut histological measurements were conducted according to the procedures used by Tran et al. (2014). Briefly, tissue samples were dehydrated and embedded in paraffin blocks. Tissue blocks were sectioned (6 µm) using the Reichert-Jung 2030 Biocut Microtome (Rankin Biomedical Corporation, Holly, MI) and collected on microscope slides, followed by removing of paraffin and staining with hematoxylin and eosin. VH, crypt depth (CD), VH to CD ratio (VH:CD), and villus surface area of duodenum and ileum were measured using Cell Sense standard software (Olympus, Center Valley, PA). Ten measurements were taken from each sample, and the means were used for statistical analysis.
Fecal VFA
Fecal samples (one pig per pen) on days 0, 14, and 21 were determined for concentrations of VFA (Kerr et al., 2015). Briefly, 1 g of feces was diluted with 5 mL of distilled water in a 15-mL polypropylene tube, followed by shaking at high speed overnight on an Eberbach Shaker (Eberbach Corp., Ann Arbor, MI). In addition, with 100 μL of phosphoric acid (final pH = 2.0 to 2.5), the tube was vortexed and centrifuged at 21,000 × g for 23 min at 4 °C. Samples were filtered through a 0.2-μm syringe filter. One milliliter of the filtrate and 0.3 g NaCl were added to a gas chromatographic vial, followed by 15 min of incubation at 70 °C. Finally, fecal VFA were analyzed using solid phase micro-extraction (silica fiber coated with Carbowax/PEG; Supelco Analytical, Bellefonte, PA) with 5 min of extraction time. The gas chromatography system was installed with a flame ionization detector and HP-FFAP column (model 7890A, column 30 m × 0.25 mm × 0.25 µm; Agilent Technologies, Wilmington, DE). The parameters used in the analysis were as follows: split mode, 30 mL/min; 24.566 psi; initial oven temperature, 100 °C; 2 min ramp of 10 °C/min to a final temperature of 240 °C.
Statistical analysis
All data were analyzed as a completely randomized design using GLIMMIX procedure (SAS Inst. Inc., Cary, NC). Pen was the experimental unit and considered a random effect for all variables. Data were analyzed as 2 × 2 factorial for the comparison of MOS and LM. The model included MOS, LM, and MOS × LM as fixed effects for growth performance, mucosal, gut histological, and VFA variables. The serum immune variables were analyzed using repeated measures, in which time, MOS × time, LM × time, and MOS × LM × time were included in the model. The serum IL-1β values were also analyzed with covariance analysis using initial IL-1β as a covariate. All means were presented as least-squares means (± SEM). A P-value no more than 0.05 was considered significant, and 0.05 < P ≤ 0.10 was denoted as a trend.
Results
Growth performance and body temperature
Growth performance data are presented in Table 2. All pigs in this study were observed with loose stool during the first week. In the period days 0 to 7, pigs fed with LM and MOS+LM had a negative ADG. As pigs recovered during the second week, data from the first 2 wk were combined and statistical analyses were performed. From days 0 to 14, the LM-treated groups had decreased (P < 0.05) G:F compared with non-LM-treated groups; however, ADG and ADFI were not different (P > 0.10) among treatments. There was an observed interaction (P < 0.05) between MOS and LM in the G:F of pigs at days 14 to 21. From days 14 to 21, G:F of pigs fed LM was greater compared with MOS+LM (P < 0.05) and control (P < 0.10), but was not different (P > 0.10) from pigs fed MOS. After pigs were fed a common diet in Phase III, G:F of pigs in LM vs. non-LM groups was lower (P < 0.05) from days 21 to 28 and tended to be lower (P < 0.10) from days 28 to 35. In contrast, the LM treatment groups had increased (P ≤ 0.05) ADFI compared with non-LM treatment groups between days 28 and 35. Combining the last 2 wk (days 21 to 35), ADG of pigs was not different (P > 0.10), but G:F of pigs was lower (P < 0.05) in LM vs. non-LM treatment groups.
Table 2.
Effects of feeding mannan oligosaccharides (MOS) and Lactobacillus mucosae (LM) on performance of weanling pigs challenged with Escherichia coli lipopolysaccharides (LPS)1
| Treatment1 | P-values | |||||||
|---|---|---|---|---|---|---|---|---|
| Item2 | Control | MOS | LM | MOS+LM | SEM3 | MOS | LM | MOS × LM |
| BW, kg | ||||||||
| Day 0 | 5.89 | 5.88 | 5.89 | 5.87 | 0.18 | 0.927 | 0.991 | 0.980 |
| Day 7 | 6.02 | 5.97 | 5.79 | 5.70 | 0.20 | 0.750 | 0.240 | 0.926 |
| Day 14 | 7.82 | 7.85 | 7.88 | 7.69 | 0.34 | 0.813 | 0.889 | 0.767 |
| Day 21 | 9.67 | 10.19 | 10.19 | 9.65 | 0.49 | 0.744 | 0.722 | 0.205 |
| Day 35 | 18.32 | 18.14 | 18.82 | 17.96 | 1.16 | 0.655 | 0.892 | 0.768 |
| Pre-LPS challenge | ||||||||
| Day 0 to 7 | ||||||||
| ADG, g | 17.98 | 13.09 | -15.36 | -24.41 | 20 | 0.737 | 0.105 | 0.920 |
| ADFI, g | 102.68 | 100.12 | 127.86 | 129.17 | 11 | 0.968 | 0.096 | 0.900 |
| G:F, g/kg | — | — | — | — | — | — | — | — |
| Days 7 to 14 | ||||||||
| ADG, g | 258.10 | 267.74 | 298.81 | 284.64 | 28 | 0.939 | 0.338 | 0.687 |
| ADFI, g | 306.49 | 303.69 | 325.71 | 328.10 | 24 | 0.993 | 0.376 | 0.915 |
| G:F, g/kg | 841.61 | 877.64 | 918.71 | 861.44 | 37 | 0.841 | 0.567 | 0.385 |
| Days 0 to 14 | ||||||||
| ADG, g | 138.04 | 140.41 | 141.72 | 130.12 | 16 | 0.780 | 0.841 | 0.672 |
| ADFI, g | 204.58 | 201.90 | 226.78 | 228.63 | 17 | 0.981 | 0.178 | 0.897 |
| G:F, g/kg | 668.34ab | 686.96a | 627.97ab | 561.48b | 36 | 0.523 | 0.042 | 0.265 |
| Post-LPS challenge | ||||||||
| Days 14 to 21 | ||||||||
| ADG, g | 271.57 | 339.39 | 350.82 | 292.14 | 35 | 0.898 | 0.654 | 0.094 |
| ADFI, g | 440.83 | 502.59 | 487.37 | 482.90 | 38 | 0.460 | 0.727 | 0.395 |
| G:F, g/kg | 615.09ab | 673.85ab | 715.41a | 599.76b | 38 | 0.466 | 0.735 | 0.040 |
| Days 21 to 28 | ||||||||
| ADG, g | 571.79 | 541.79 | 573.87 | 523.27 | 29 | 0.196 | 0.785 | 0.733 |
| ADFI, g | 781.70 | 752.50 | 829.29 | 803.33 | 41 | 0.517 | 0.257 | 0.969 |
| G:F, g/kg | 731.40a | 719.53a | 693.78ab | 651.50b | 16 | 0.119 | 0.007 | 0.364 |
| Days 28 to 35 | ||||||||
| ADG, g | 648.04 | 589.88 | 626.67 | 649.58 | 34 | 0.619 | 0.588 | 0.262 |
| ADFI, g | 993.93ab | 884.59b | 1,029.29ab | 1,039.70a | 50 | 0.342 | 0.050 | 0.254 |
| G:F, g/kg | 650.71 | 665.42 | 612.12 | 625.82 | 22 | 0.525 | 0.097 | 0.982 |
| Days 21 to 35 | ||||||||
| ADG, g | 609.91 | 565.83 | 600.27 | 586.43 | 28 | 0.316 | 0.846 | 0.595 |
| ADFI, g | 887.81 | 818.54 | 929.28 | 921.52 | 42 | 0.382 | 0.114 | 0.482 |
| G:F, g/kg | 686.50ab | 690.69a | 648.55ab | 636.80b | 17 | 0.827 | 0.019 | 0.647 |
| Days 0 to 35 | ||||||||
| ADG, g | 355.29 | 348.33 | 363.51 | 349.05 | 18 | 0.564 | 0.809 | 0.839 |
| ADFI, g | 470.30 | 450.76 | 483.46 | 481.78 | 23 | 0.657 | 0.361 | 0.708 |
| G:F, g/kg | 754.99 | 774.81 | 751.70 | 726.71 | 24 | 0.915 | 0.301 | 0.364 |
1Supplementation of 0.1% of MOS in the diet and approximately 109 cfu of LM per pig was done in Phase I (days 1 to 14) and Phase II (days 15 to 28). The n = 4 replicates per treatment; there were six pigs per pen from days 0 to 14, five pigs per pen from days 14 to 21, and four pigs per pen from days 21 to 35.
2ADFI, average daily feed intake; ADG, average daily gain; BW, body weight; G:F, gain-to-feed ratio; LPS, lipopolysaccharide.
3Pooled standard error of the treatment means.
a,bMeans within rows with different superscript are different at P ≤ 0.05.
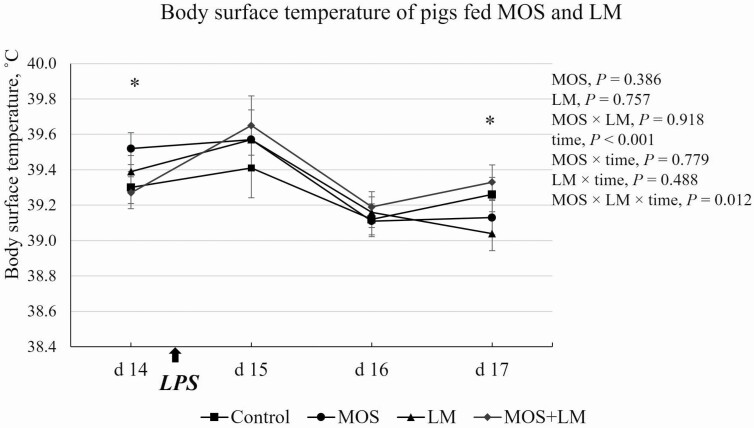
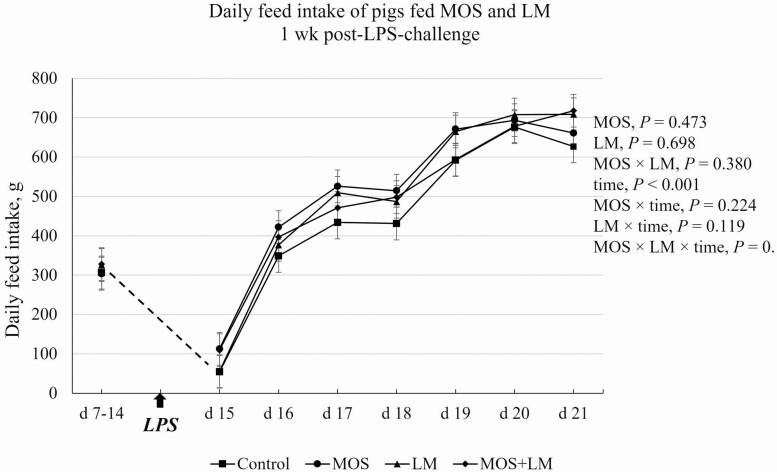
The LPS challenge on day 14 caused increased (P < 0.05) body surface temperature on day 15, but the temperature immediately decreased (P < 0.05) on day 16 (Figure 1). There was an MOS × LM × time interaction (P < 0.05) in body temperature of pigs. On day 14, pigs fed MOS tended to (P < 0.10) have greater body temperature than pigs fed control and MOS+LM diets. No differences (P > 0.10) were observed for body temperature of pigs on days 15 and 16. On day 17, body temperature was decreased (P < 0.05) in pigs fed LM vs. MOS+LM group. The daily feed intake of pigs recovered within 2 d post-LPS challenge (Figure 2). There was a tendency of MOS × LM × time interaction (P < 0.10), but no significant main effects (P > 0.10) in ADFI were observed between dietary treatments.
Figure 1.
Effects of feeding mannan oligosaccharides (MOS) and Lactobacillus mucosae (LM) on body surface temperature of pigs challenged with Escherichia coli lipopolysaccharides (LPS). Supplementation of 0.1% of MOS in the diet and approximately 109 cfu of LM per pig was done in Phase I (days 1 to 14) and Phase II (days 15 to 28). *On day 14, MOS vs. control and MOS+LM, P < 0.10; on day 17, LM vs. MOS+LM, P < 0.05.
Figure 2.
Effect of feeding mannan oligosaccharides (MOS) and Lactobacillus mucosae (LM) on daily feed intake of pigs 1 wk after challenged with Escherichia coli lipopolysaccharides (LPS). Supplementation of 0.1% of MOS in the diet and approximately 109 cfu of LM per pig was done in Phase I (days 1 to 14) and Phase II (days 15 to 28).
Serum and mucosal immunoglobulins and IL-1β
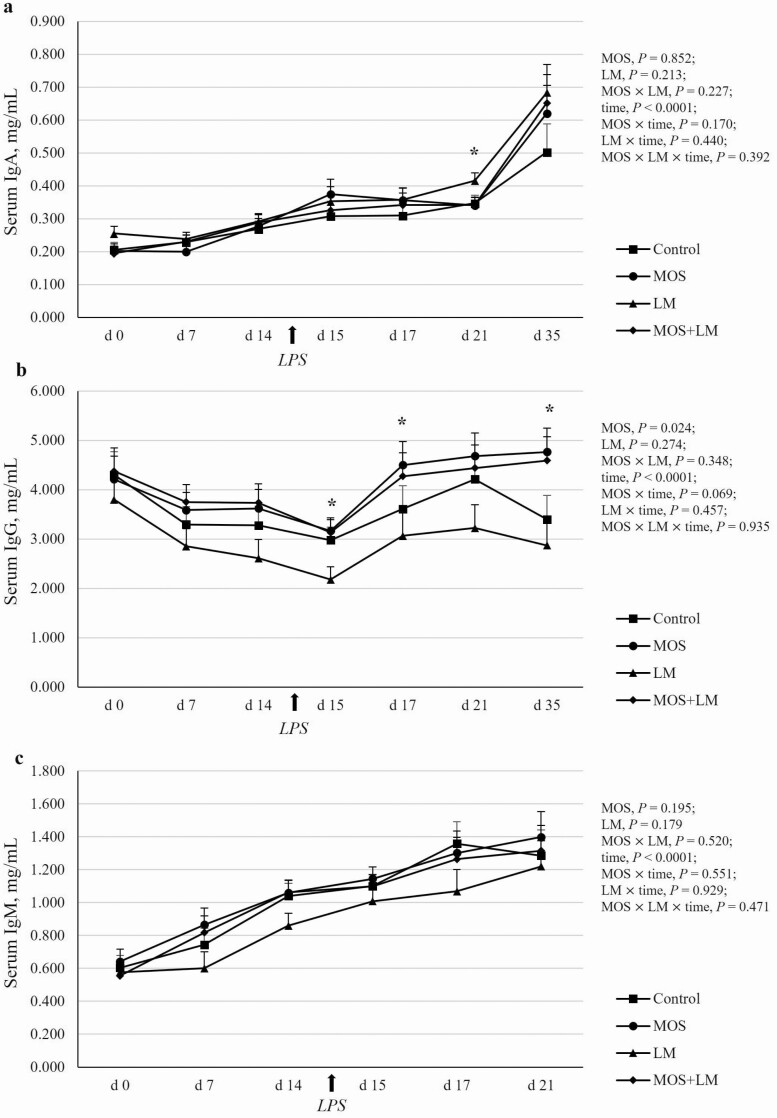
The time effects were significant (P < 0.05) for serum IgA, IgG, and IgM (Figure 3). Circulating IgA and IgM increased (P < 0.05) over time (Figure 3a and c), while IgG decreased (P < 0.05) from weaning to day 1 postchallenge (day 15) and increased (P < 0.05) thereafter (Figure 3b). A significant MOS effect (P < 0.05) and a tendency of MOS × time effect (P < 0.10) was observed for circulating IgG concentrations. Feeding MOS vs. non-MOS-treated diets increased (P < 0.05) serum IgG on days 15 and 17 postweaning. After removal of dietary treatments for 2 wk (day 35), the MOS treatment groups showed increased (P < 0.05) serum IgG concentrations compared with non-MOS treatment groups. There was no significant treatment or treatment by time interaction effects for circulating IgA. Nevertheless, on day 21, serum IgA was increased (P < 0.05) in pigs fed LM compared with MOS and MOS+LM groups and tended to be greater (P < 0.10) in LM vs. control-fed pigs. Dietary treatment did not affect (P > 0.10) circulating IgM of pigs.
Figure 3.
Effects of feeding mannan oligosaccharides (MOS) and Lactobacillus mucosae (LM) on circulating immunoglobulins in weanling pigs challenged with Escherichia coli lipopolysaccharides (LPS). Supplementation of 0.1% of MOS in the diet and approximately 109 cfu of LM per pig was done in Phase I (days 1 to 14) and Phase II (days 15 to 28). N = 4 pens per treatment. All pigs were challenged with LPS after the blood collection on day 14. There were four samples per pen from days 0 to 21 and two samples per pen on day 35. The serum immunoglobulins were measured using commercial porcine-specific ELISA kits. The time effects were significant (P < 0.05) for all three immunoglobulins. (a) Serum IgA concentrations. No treatment or treatment by time effects were observed (P > 0.10). *On day 21, LM vs. MOS and MOS+LM, P < 0.05. (b) Serum IgG concentrations. *Main effects (P < 0.05) of MOS were observed on days 15, 17, and 35, respectively; on days 15 and 35, respectively, MOS and MOS+LM vs. LM, P < 0.05. (c) Serum IgM concentrations. No treatment or treatment by time effects were observed (P > 0.10).
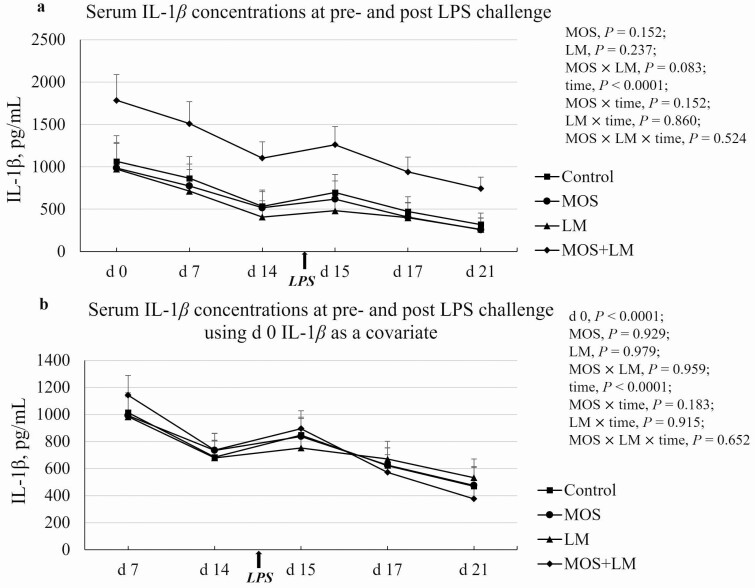
Serum IL-1β concentration decreased (P < 0.05) from days 0 to 14 (1,202 to 640 pg/mL), increased on day 15 (P < 0.05; 765 pg/mL), and continuously dropped until day 21 (396 pg/mL; Figure 4a). There was a tendency of MOS × LM interaction (P < 0.10) in circulating IL-1β concentration. On day 0, IL-1β tended to be greater (P < 0.10) in MOS+LM vs. MOS and LM groups. On days 7, 15, and 17, IL-1β of pigs fed MOS+LM was increased (P < 0.05) compared with LM and tended to be greater (P < 0.10) compared to control and MOS groups. On day 14, IL-1β was greater (P < 0.05) in pigs fed MOS+LM vs. MOS and LM and tended to be greater (P < 0.10) in MOS+LM vs. control. On day 21, pigs fed MOS+LM had the greatest (P < 0.05) IL-1β concentration compared with other groups. Using day 0 (P < 0.05), IL-1β concentration as a covariate (Figure 4b), there were no differences (P > 0.10) among dietary treatments at any other time point. However, circulating IL-1β in control and MOS+LM pigs were increased (P < 0.05) on day 15 compared with pre-LPS challenge, but were not affected (P > 0.10) in pigs fed MOS or LM diet.
Figure 4.
Effect of feeding mannan oligosaccharides (MOS) and Lactobacillus mucosae (LM) on circulating IL-1β concentrations in weanling pigs challenged with Escherichia coli lipopolysaccharides (LPS). Supplementation of 0.1% of MOS in the diet and approximately 109 cfu of LM per pig was done in Phase I (days 1 to 14) and Phase II (days 15 to 28). (a) Serum IL-1β concentrations. b. Serum IL-1β data plot using day 0 IL-1β concentration as a covariate. n = 4 pens per treatment. All pigs were challenged with LPS after the blood collection on day 14.
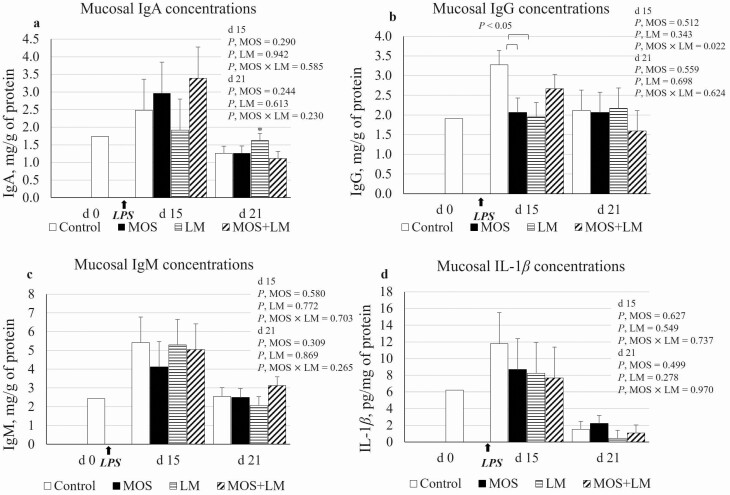
The secretory IgA, IgG, and IgM are presented as mg/g of protein and mucosal IL-1β is presented as pg/mg of protein (Figure 5). There was an MOS × LM interaction (P < 0.05) in secretory IgG concentration on day 15; mucosal IgG was increased (P < 0.05) in control compared with MOS and LM groups but was not different (P > 0.10) from MOS+LM. However, there were no differences (P > 0.10) in secretory IgG among dietary treatments on day 21. No differences (P > 0.10) were observed for secretory IgA, IgM, or IL-1β concentrations. On day 21, a contrast analysis showed a tendency of increased (P < 0.10) mucosal IgA in pigs fed LM compared with MOS+LM.
Figure 5.
Effect of feeding mannan oligosaccharides (MOS) and Lactobacillus mucosae (LM) on ileal mucosal immunoglobulins and IL-1β concentrations in weanling pigs challenged with Escherichia coli lipopolysaccharides (LPS). Supplementation of 0.1% of MOS in the diet and approximately 109 cfu of LM per pig was done in Phase I (days 1 to 14) and Phase II (days 15 to 28). a. mucosal IgA; *on day 21, LM vs. MOS+LM: P < 0.10; b. mucosal IgG; c. mucosal IgM; d. mucosal IL-1β; on day 21, 10 out of 16 IL-1β measurements were not detectable and were considered 0 pg/mg of protein. n = 4 pens per treatment.
Small intestine histological analyses
The duodenal and ileal histological data of pigs are presented in Table 3. On day 15, feeding LM tended (P < 0.10) to decrease CD in the ileum; pigs fed LM had decreased ileal CD (P < 0.05) compared with control, but were not different (P > 0.10) from the pigs fed MOS or MOS+LM diets. However, all other ileal and duodenal measurements on days 15 and 21 were not different (P > 0.10) among treatments.
Table 3.
Effects of feeding mannan oligosaccharides (MOS) and Lactobacillus mucosae (LM) on small intestine histological analyses in weanling pigs challenged with Escherichia coli lipopolysaccharides (LPS)1
| Treatment | P-values | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameters2 | Control | MOS | LM | MOS+LM | SEM3 | MOS | LM | MOS × LM |
| Day 15 duodenum | ||||||||
| VH, µm | 321.9 | 344.2 | 368.3 | 339.5 | 35 | 0.929 | 0.565 | 0.483 |
| CD, µm | 360.4 | 319.2 | 374.1 | 371.9 | 34 | 0.530 | 0.342 | 0.573 |
| VH:CD | 0.9 | 1.1 | 1.1 | 0.9 | 0.1 | 0.888 | 0.907 | 0.202 |
| Villus area, µm2 | 36,350 | 41,504 | 43,979 | 39,543 | 5,962 | 0.953 | 0.643 | 0.437 |
| Day 15 ileum | ||||||||
| VH, µm | 329.5 | 312.2 | 354.5 | 289.8 | 32 | 0.225 | 0.968 | 0.474 |
| CD, µm | 288.0b | 266.9 | 245.6a | 258.6 | 14 | 0.773 | 0.091 | 0.239 |
| VH:CD | 1.2 | 1.2 | 1.5 | 1.2 | 0.2 | 0.312 | 0.442 | 0.303 |
| Villus area, µm2 | 38,023 | 36,240 | 42,575 | 32,523 | 5,330 | 0.289 | 0.939 | 0.453 |
| Day 21 duodenum | ||||||||
| VH, µm | 492.9 | 573.8 | 522.0 | 543.9 | 35 | 0.163 | 0.991 | 0.411 |
| CD, µm | 478.8 | 469.3 | 460.9 | 424.9 | 30 | 0.465 | 0.321 | 0.669 |
| VH:CD | 1.1 | 1.3 | 1.2 | 1.3 | 0.1 | 0.135 | 0.604 | 0.894 |
| Villus area, µm | 75,077 | 92,409 | 81,651 | 82,906 | 7,212 | 0.222 | 0.843 | 0.287 |
| Day 21 ileum | ||||||||
| VH, µm | 469.0 | 427.3 | 481.9 | 444.8 | 28 | 0.191 | 0.603 | 0.938 |
| CD, µm | 303.0 | 284.8 | 272.5 | 289.4 | 23 | 0.976 | 0.587 | 0.465 |
| VH:CD | 1.7 | 1.6 | 1.9 | 1.6 | 0.1 | 0.308 | 0.424 | 0.494 |
| Villus area, µm2 | 62,241 | 56,517 | 62,216 | 55,510 | 5,329 | 0.266 | 0.925 | 0.928 |
1Supplementation of 0.1% of MOS in the diet and approximately 109 cfu of LM per pig was done in Phase I (days 1 to 14) and Phase II (days 15 to 28). The n = 4 pens per treatment; one pig per pen was euthanized for collection of samples on days 15 and 21 to analyze histological parameters.
2Histological parameters were measured following standard staining and paraffin embedding procedures of tissue, and Cell Sense software. CD, crypt depth; VH, villus height; VH:CD, villus height to crypt depth ratio.
3Pooled standard error of the treatment means.
a,bMeans within rows with different superscript are different at P ≤ 0.05.
Fecal VFA
Effects of MOS and LM on concentration of fecal VFA before and after LPS challenge are shown in Table 4. A total of nine VFA were analyzed, but many of the values for isocaproate, caproate, and heptanoate were below the lowest standard concentrations and are not presented here. On days 0 and 14, there were no differences (P > 0.10) in concentrations of VFA. On day 21, supplementation of MOS and LM tended (P < 0.10) to have interaction effect in propionate; feeding MOS tended (P < 0.10) to decrease propionate compared with control and MOS+LM group but was not different (P > 0.10) from LM-fed pigs. Main effects or interaction effects (P > 0.10) were not observed for concentrations of other VFA on day 21. However, a contrast analysis showed that pigs fed MOS tended to have lower (P < 0.10) butyrate compared with control and MOS+LM-fed pigs.
Table 4.
Effects of feeding mannan oligosaccharides (MOS) and Lactobacillus mucosae (LM) on fecal concentrations of VFA in weanling pigs challenged with Escherichia coli lipopolysaccharides (LPS; as is basis, mM)1
| Treatment | P-values | |||||||
|---|---|---|---|---|---|---|---|---|
| Items | Control | MOS | LM | MOS+LM | SEM2 | MOS | LM | MOS × LM |
| Acetate | ||||||||
| Day 0 | 1.47 | 0.77 | 1.78 | 0.34 | 0.84 | 0.246 | 0.945 | 0.685 |
| Day 14 | 21.92 | 26.55 | 22.86 | 20.24 | 7.61 | 0.897 | 0.730 | 0.642 |
| Day 21 | 17.50 | 32.15 | 21.56 | 19.48 | 8.87 | 0.493 | 0.636 | 0.364 |
| Propionate | ||||||||
| Day 0 | 0.147 | 0.159 | 0.115 | 0.090 | 0.05 | 0.905 | 0.382 | 0.745 |
| Day 14 | 6.886 | 5.556 | 6.738 | 5.671 | 1.51 | 0.443 | 0.991 | 0.932 |
| Day 21 | 6.773 | 3.745 | 5.885 | 6.792 | 1.01 | 0.315 | 0.306 | 0.075 |
| Isobutyrate | ||||||||
| Day 0 | 0.219 | 0.294 | 0.245 | 0.193 | 0.07 | 0.875 | 0.618 | 0.411 |
| Day 14 | 0.620 | 0.508 | 0.711 | 0.843 | 0.17 | 0.953 | 0.241 | 0.493 |
| Day 21 | 0.474 | 0.471 | 0.749 | 0.596 | 0.13 | 0.545 | 0.137 | 0.563 |
| Butyrate | ||||||||
| Day 0 | 0.079 | 0.067 | 0.343 | 0.046 | 0.17 | 0.396 | 0.502 | 0.434 |
| Day 14 | 3.497 | 1.783 | 3.449 | 3.541 | 0.92 | 0.398 | 0.374 | 0.348 |
| Day 21 | 3.480 | 2.117 | 3.222 | 3.421 | 0.45 | 0.224 | 0.272 | 0.111 |
| Isovalerate | ||||||||
| Day 0 | 0.659 | 0.579 | 0.715 | 0.560 | 0.13 | 0.416 | 0.895 | 0.793 |
| Day 14 | 0.992 | 0.745 | 1.065 | 1.370 | 0.29 | 0.920 | 0.246 | 0.354 |
| Day 21 | 0.800 | 0.659 | 1.219 | 1.038 | 0.27 | 0.557 | 0.160 | 0.942 |
| Valerate | ||||||||
| Day 0 | 0.072 | 0.088 | 0.077 | 0.054 | 0.03 | 0.918 | 0.641 | 0.552 |
| Day 14 | 1.146 | 0.708 | 0.857 | 0.936 | 0.24 | 0.462 | 0.901 | 0.295 |
| Day 21 | 1.044 | 0.799 | 0.976 | 1.293 | 0.22 | 0.871 | 0.354 | 0.228 |
| Total VFA | ||||||||
| Day 0 | 2.66 | 1.96 | 3.27 | 1.29 | 1.14 | 0.284 | 0.985 | 0.601 |
| Day 14 | 35.18 | 35.95 | 35.79 | 32.79 | 9.90 | 0.912 | 0.899 | 0.852 |
| Day 21 | 30.26 | 40.09 | 33.81 | 32.86 | 8.43 | 0.608 | 0.831 | 0.535 |
1Supplementation of 0.1% of MOS in the diet and approximately 109 cfu of LM per pig was done in Phase I (days 1 to 14) and Phase II (days 15 to 28). The n = 4 fecal samples per treatment (one pig per pen) were collected via rectal massage on days 0, 14, and 21 and analyzed for volatile fatty acids using gas chromatography.
2Pooled standard error of the treatment means.
The amounts of individual VFA in proportion of total VFA were calculated (Table 5). There were no differences (P > 0.10) on day 0. However, on day 14, pigs fed MOS and LM had interaction effects in percentage of acetate (P < 0.10), butyrate (P < 0.05), and valerate (P < 0.10). Pigs fed MOS had greater (P < 0.05) acetate, but lower (P < 0.05) butyrate and valerate in proportion of total VFA compared with control group. In addition, pigs fed MOS tended (P < 0.10) to have greater acetate compared with MOS+LM group. The proportions of isobutyrate (P < 0.10), butyrate (P < 0.05), and isovalerate (P < 0.10) were lower in MOS vs. MOS+LM group. On day 21, no significant (P > 0.10) main effects or interaction effect were observed but feeding MOS tended to decrease (P < 0.10) the proportions of propionate and butyrate compared with pigs fed the control diet.
Table 5.
Effects of feeding mannan oligosaccharides (MOS) and Lactobacillus mucosae (LM) on fecal volatile fatty acids (VFA) ratios in weanling pigs challenged with Escherichia coli lipopolysaccharides (LPS; % of total VFA)1
| Treatment | P-values | |||||||
|---|---|---|---|---|---|---|---|---|
| VFA | Control | MOS | LM | MOS+LM | SEM2 | MOS | LM | MOS × LM |
| Acetate | ||||||||
| Day 0 | 41.60 | 33.12 | 41.00 | 25.85 | 10.59 | 0.307 | 0.728 | 0.768 |
| Day 14 | 55.83ab | 72.91a | 62.77ab | 55.11b | 5.71 | 0.425 | 0.360 | 0.051 |
| Day 21 | 56.19 | 71.32 | 61.02 | 56.88 | 7.04 | 0.450 | 0.508 | 0.197 |
| Propionate | ||||||||
| Day 0 | 5.62 | 9.94 | 6.34 | 6.82 | 2.10 | 0.296 | 0.594 | 0.398 |
| Day 14 | 20.33 | 16.02 | 19.84 | 21.36 | 2.81 | 0.627 | 0.404 | 0.319 |
| Day 21 | 22.97 | 14.15 | 18.95 | 21.19 | 3.34 | 0.345 | 0.660 | 0.124 |
| Isobutyrate | ||||||||
| Day 0 | 11.11 | 17.52 | 9.97 | 14.28 | 3.25 | 0.141 | 0.530 | 0.763 |
| Day 14 | 2.70 | 1.49 | 2.16 | 3.32 | 0.69 | 0.972 | 0.367 | 0.113 |
| Day 21 | 1.77 | 1.64 | 2.45 | 2.07 | 0.61 | 0.681 | 0.381 | 0.835 |
| Butyrate | ||||||||
| Day 0 | 2.89 | 3.95 | 4.90 | 3.65 | 1.69 | 0.960 | 0.638 | 0.526 |
| Day 14 | 12.07a | 5.06b | 9.08ab | 11.11a | 1.77 | 0.185 | 0.405 | 0.025 |
| Day 21 | 12.05 | 7.32 | 9.99 | 11.29 | 1.86 | 0.374 | 0.617 | 0.131 |
| Isovalerate | ||||||||
| Day 0 | 34.95 | 30.69 | 35.42 | 43.75 | 8.20 | 0.816 | 0.445 | 0.476 |
| Day 14 | 4.65 | 2.15 | 3.36 | 5.24 | 1.24 | 0.808 | 0.480 | 0.103 |
| Day 21 | 2.91 | 2.36 | 4.10 | 3.55 | 1.14 | 0.638 | 0.317 | 0.997 |
| Valerate | ||||||||
| Day 0 | 3.57 | 4.52 | 2.21 | 4.68 | 1.47 | 0.288 | 0.702 | 0.633 |
| Day 14 | 3.96a | 2.05b | 2.48ab | 3.21ab | 0.62 | 0.352 | 0.798 | 0.053 |
| Day 21 | 3.53 | 2.74 | 2.96 | 4.18 | 0.70 | 0.767 | 0.543 | 0.176 |
1Supplementation of 0.1% of MOS in the diet and approximately 109 cfu of LM per pig was done in Phase I (days 1 to 14) and Phase II (days 15 to 28). The n = 4 fecal samples treatment (one pig per pen) were collected via rectal massage on days 0, 14, and 21 and analyzed for volatile fatty acids using gas chromatography.
2Pooled standard error of the treatment means.
a,bUsed for treatment comparisons. Means within rows are different at P ≤ 0.05.
Discussion
MOS derived from inactivated yeast (Saccharomyces cerevisiae), containing at least 37% of glucomannan complex, was used in early nursery diets to improve immune indicators of pigs under weanling stress and inflammatory challenge. It has been reported that supplementation of MOS increased growth performance and nutrient digestibility in weanling pigs (Zhao et al., 2012). In addition, our preliminary study (data not published) showed that feeding MOS increased fecal population of LM, a potential probiotic strain (Valeriano et al., 2014). In the current study, we successfully isolated a pure LM strain from fresh fecal samples of nursery pigs. We evaluated the effects of feeding MOS, LM, and the combination of both on growth performance, immune response, and gut health of weanling pigs challenged with E. coli LPS.
Growth performance and body temperature
The growth performance of the pigs during the first week was not statistically different, but a negative ADG was observed in pigs given LM and MOS+LM. The negative growth rate on day 7 might be due to the disturbance in microbial balance in the gut of pigs supplemented with LM. With this disturbance, it may have affected the ability of the pig to utilize feed materials and contributed to reduced growth rate. Microbial balance has a role in the development and maturation of the gut (Li et al., 2021), which is essential in weanling pigs. Microbes, such as LM, participate in the digestion, absorption, and physiological functions of the gut (Fan and Pederson, 2021). This may also imply that the first week of supplementation may have been an adjustment period for the pigs and allow for the integration of the introduced bacteria in the gut. In the current study, feeding MOS increased G:F compared with MOS+LM during the first 2 wk of the experiment. This was inconsistent with our previous study, which showed decreased G:F of pigs fed MOS diet (data not published). It may be due to the different health conditions of the pigs. In the present study, pigs had diarrhea during the first 2 wk postweaning, while pigs in the previous study had essentially no diarrhea issues. The improved G:F by dietary MOS diminished during the first week of LPS challenge. However, pigs fed LM showed increased G:F compared with control and MOS+LM pigs from days 14 to 21, indicating that supplementation of LM may protect the pigs against acute inflammation due to LPS challenge. From days 21 to 35, pigs were recovered from LPS challenge and were fed a common diet; subsequently, the beneficial effects from feeding LM disappeared. Feeding L. mucosae directly to pigs has not been previously studied. In an E. coli challenge study, inclusion of Lactobacillus plantarum showed improved growth performance of weanling pigs (Lee et al., 2012a). Improved gain in weight in LPS-challenged pigs was observed in pigs supplemented with Lactobacillu salivarius (Sun et al., 2020). The MOS+LM pigs had the lowest growth performance throughout the experiment, indicating that feeding MOS and LM may not have synergistic effects on growth performance of weanling pigs. In a study in chickens, the MOS were poorly utilized by Lactobacillus strains and growth studies showed that variations in growth rates may be due to the ability of the probiotic strain to utilize the substrate, which is important in selecting suitable prebiotic oligosaccharide for the preparation of synbiotics (Saminathan et al., 2011). Fibers that are poorly utilized by gut microbes increase the rate of digesta transit and reduce the time available for microbial fermentation (Titgemeyer et al., 1991). This may reduce nutrient digestibility and ability of growing animals to ferment dietary fiber (Gutierrez et al., 2013), and this may explain the unfavorable effect of MOS+LM on growth.
The ear base is one of the best locations for skin temperature recording that highly correlated with rectal temperature (Soerensen and Pedersen, 2015).Therefore, skin temperature changes during LPS challenge were used to represent the trend of body temperature in the current study. The slight increase of skin temperature on day 15 indicated that acute inflammatory responses occurred within 2 d post-LPS challenge. This was also in agreement with the results of daily feed intake during the first week of challenge, as the feed intake of pigs recovered 2 day postchallenge. Similar observations in feed intake were reported in pigs after LPS challenge (Campos et al., 2014). Fermentation products of Lactobacillus acidophilus have been observed to reduce acute phase responses in nursery pigs following LPS challenge (Sanchez et al., 2019). The lowest skin temperature of pigs fed LM on day 17, indicated that feeding LM may accelerate the recovery of pigs from LPS challenge.
Serum and mucosal immunoglobulins and IL-1β
The mucosal immune system is essential for producing immunoglobulins (Ohland and MacNaughton, 2010). At the mucosal effector sites, plasma B cells are activated by antigenic stimulation and differentiate into cells that produce specific class of antibodies (IgA, IgG, IgM, etc.) against infections (Abbas et al., 2014). Mucosa epithelial cells secrete IgA, which helps neutralize microbes in the lumen of respiratory and gastrointestinal tracts (Kaetzel, 2014). The IgG antibodies, representing 20% of animal plasma, are generated by class switching (i.e., IgM to IgG) from plasma B cells (Butler et al., 2006). The IgG and IgM promote phagocytosis of microbes by activating the complement system (Yoshida et al., 2006; Abbas et al., 2014). In the current study, circulating but not secretory IgG antibodies were increased by feeding MOS diets, indicating that MOS may promote class switching of B cells to IgG in the portal vein. Based on the result of this initial study, there is not enough evidence to prove that class switching IgG in the gut mucosa is stimulated by MOS. In addition, MOS seems to have a carryover effect on circulating IgG concentrations after removal of treatments for 2 wk. This is likely because the IgG molecules have a half-life of 21 to 28 d, whereas the half-life of IgA is only approximately 3 d (Abbas et al., 2014). Hinkle (2012) showed that circulating IgA and IgG peaked on day 35 postweaning. The increased IgG concentration by feeding MOS may suggest an earlier maturation of immune system in weanling pigs. Additionally, the mucosal IgG was increased in control pigs immediately after LPS challenge, indicating that the production of IgG was necessary for attenuating acute inflammation.
However, feeding LM alone had a mild increase in both secretory and circulating IgA concentrations of pigs after 3 wk of treatment. This result was in agreement with our previous multivariate analysis, which showed a positive correlation between abundance of fecal LM and circulating IgA concentration (data not published). Zhang et al. (2010) reported increased secretory IgA in pigs challenged with E. coli K88 and feeding Lactobacillus rhamnosus further increased the production of secretory IgA. Similar observations were reported in weaned pigs E. coli K88 that were given L. acidophilus with increased concentration of IgA in the jejunum (Li et al., 2018). Therefore, we suggest that the increased mucosal and serum IgA observed by feeding LM likely protected the pigs from acute inflammation caused by LPS challenge. However, this beneficial effect diminished after removal of the treatments for 1 wk.
The IL-1β is a proinflammatory cytokine and an indicator for acute inflammation (Medzhitov et al., 1998). In the current study, the greatest circulating IL-1β concentration appeared on day 0, indicating that pigs had an immune inflammatory activation on the weaning day. This was likely because the pigs were weaned and transported for about an hour to the experimental location before the collection of day 0 blood samples. The greatest IL-1β concentration in MOS+LM pigs indicates that these pigs maintained a greater proinflammatory status from the beginning of the experiment, which may cause the lower growth performance. During the experimental period, circulating IL-1β concentration dropped between weaning and pre-LPS challenge, but increased for approximately 120 pg/mL from prechallenge to 24 h postchallenge, followed by a continuous decrease 3 d postchallenge. When comparing the time effect within dietary treatments, the LPS challenge increased serum IL-1β in control and MOS+LM groups, but did not affect MOS and LM groups, indicating that feeding MOS or LM alone prevented the acute inflammation caused by E. coli-LPS challenge. MOS supplementation in pigs challenged with E. coli K88 had reduced concentration of IL-1β and other markers of intestinal inflammation (Yu et al., 2021). Similarly, decreased proinflammatory cytokines were observed in E. coli-LPS-challenged pigs fed levan-type fructan (Li and Kim, 2013). Supplementation of probiotic Lactobacillus species has been shown to alleviate gut inflammation, improve intestinal barrier function, and decrease proinflammatory cytokines (L. rhamnosus GG; Mao et al., 2020) and to also downregulate IL-1β (Lactobacillus fermentum; Wang et al., 2019).
Small intestine histological analyses
In the present study, feeding LM for 2 wk decreased ileal CD of weanling pigs. This reduced CD was also observed along with increased VH:CD in the jejunum and ileum of weaned pigs supplemented with Lactobacillus reuteri LR1 (Yi et al., 2018). The decreased CD is associated with increasingly differentiated crypt cells and improved gut absorptive capacity (Zhao et al., 2007), while an increased VH:CD indicates increasing nutrient digestibility and absorptive rate in the small intestine (Rubio et al., 2010). We suggest that the decreased ileal CD may indicate improved nutrient absorptive rate in the ileum, leading to the increased G:F by feeding LM during the first week of LPS challenge. In addition, the differences of histological measurements were observed in ileum, but not in duodenum, indicating that L. mucosae may primarily colonize and communicate with the mucus layer at the ileum.
Fecal VFA
In the current study, feeding MOS alone decreased the amount of propionate and butyrate (as is basis). To exclude the possible differences in DM content of individual samples, we calculated the percentage of each VFA in proportion of total VFA. The results were in agreement with as is based data, where feeding MOS showed lower proportions of propionate, butyrate, and even valerate compared with pigs fed the control diet. These differences contributed to the increase proportion of acetate in pigs fed the MOS diet. However, White et al. (2002) only observed moderate decreases of valerate and isovalerate in pigs fed MOS diet, while a previous study in our group showed increased propionate, butyrate, and total VFA by feeding MOS (data not published). Studies have shown that bacterial metabolites (i.e., propionate and butyrate) improved intestinal barrier, decreased incidences of diarrhea, and facilitated differentiation of immune cells (Zeyner and Boldt, 2006; Peng et al., 2009; Arpaia et al., 2013). In addition, the decreased butyrate production in MOS-fed pigs may also be attributed to the different gut environmental conditions. It was suggested that a reduced pH increases population of butyrate-producing bacteria, thus promoting butyrate formation (Louis and Flint, 2009). However, we did not measure pH values in this study.
The moderate change of VFA production in the pigs given LM and MOS may be due to the level of supplementation and age of the pigs. Fermentable soluble fibers may negatively affect the feed digestion as these may increase digesta viscosity resulting to impaired glucose absorption, and reduced fat emulsification (Anderson, 2009; Holscher, 2017). Mannan is insoluble in water. Insoluble fibers may increase digesta transit and reduce the ability of the animal to ferment dietary fiber (Gutierrez et al., 2013), which may explain the moderate change in VFA production. Nursery pigs have immature gut that are subjected to weaning stress resulting to reduced secretion of enzyme and substances needed for digestion (Pluske et al., 1995). Newly weaned pigs may be more susceptible to some unfavorable effects of fiber supplementation.
The level of supplementation and the age of the pigs may affect the response to MOS supplementation. In a study, pigs were given at rate of 1 g/kg of diet (0.1%) and was given to pigs 28 d of age (Nochta et al., 2010). The rate of inclusion used in the study is the same as the published literature, and the pigs were weaned at a slightly younger age (around 23 d). In terms of inclusion, supplementation of MOS to pigs between 7 and 35 d of age at a rate of 0.8 g/kg diet was studied (Duan et al., 2016), which was lower than the inclusion used in the current study (0.1%). Other studies also showed that pigs supplemented MOS were at least 21 d of age (Zhao et al., 2012; Valpotić et al, 2017; Agazzi et al, 2020). However, in a meta-analysis of published MOS trial with nursery pigs, they failed to clearly show that weaning age affected the response of the animals to MOS supplementation (Miguel et al., 2004).
Conclusions
The results of the present study indicate that feeding MOS protects weanling pigs from E. coli LPS challenge by increasing circulating IgG concentration. Supplementation of MOS may induce an earlier maturation of humoral immunity in weanling pigs. In contrast with other studies, feeding MOS increased fecal acetate, but decreased propionate and butyrate production. We propose that this was due to the different immune status and gut environmental conditions of pigs used in this study. During LPS challenge, supplementation of LM may protect the pigs against acute inflammation by 1) increasing production of ileal mucosal IgA and thereby promoting circulating IgA concentration and 2) facilitating nutrient absorptive rate by decreasing ileal CD and therefore, increasing feed efficiency. However, these beneficial effects were not observed when pigs were not challenged with LPS or after removal of treatment. There was no observed synergistic effect of MOS and LM. In summary, these preliminary findings suggest that MOS can be used as a prebiotic to promote maturation of IgG production in weanling pigs, while LM can be used as a probiotic to improve mucosal immunity especially during acute inflammation.
Acknowledgments
Authors are thankful to the staff of the swine production unit of the Eastern Nebraska Research and Extension Center. A contribution of the University of Nebraska Agricultural Resource Division, supported in part by funds provided through the Hatch Act. Mention of a trade name, proprietary product, or specific equipment does not constitute a guarantee or warranty by the USDA or the University of Nebraska and does not imply approval to the exclusion of other products that may be suitable. The USDA is an equal opportunity provider and employer.
Glossary
Abbreviations
- ADFI
average daily feed intake
- ADG
average daily gain
- BLAST
Basic Local Alignment Search Tool
- BW
body weight
- CD
crypt depth
- cfu
colony forming unit
- ELISA
enzyme linked immunosorbent assay
- G:F
gain-to-feed ratio
- IgA
immunoglobulin A
- IgG
immunoglobulin G
- IgM
immunoglobulin M
- IL-1β
interleukin beta
- LPS
lipopolysaccharide
- MOS
mannan oligosaccharide
- MRS
De Man, Rogosa and Sharpe
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- VFA
volatile fatty acids
- VH
villus height
- VH:CD
villus height to crypt depth ratio
Conflict of interest statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Abbas, A. K., Lichtman A. H., and Pillai S.. . 2014. Cellular and molecular immunology. Philadelphia, PA: Elsevier Health Sciences. [Google Scholar]
- Agazzi, A., Perricone V., Omodei Zorini F., Sandrini S., Mariani E., Jiang X. R., Ferrari A., Crestani M., Nguyen T. X., Bontempo V., . et al. 2020. Dietary mannan oligosaccharides modulate gut inflammatory response and improve duodenal villi height in post-weaning piglets improving feed efficiency. Animals 10:1283. doi: 10.3390/ani10081283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, J. W., Baird P., R. H.Davis, Jr., Ferreri S., Knudtson M., Koraym A., Waters V., and Williams C. L.. . 2009. Health benefits of dietary fiber. Nutr. Rev. 67:188–205. doi: 10.1111/j.1753-4887.2009.00189.x [DOI] [PubMed] [Google Scholar]
- Arpaia, N., Campbell C., Fan X., Dikiy S., van der Veeken J., deRoos P., Liu H., Cross J. R., Pfeffer K., Coffer P. J., . et al. 2013. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504:451–455. doi: 10.1038/nature12726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkey, T. E., Skjolaas K. A., and Minton J. E.. . 2009. Board-invited review: porcine mucosal immunity of the gastrointestinal tract. J. Anim. Sci. 87:1493–1501. doi: 10.2527/jas.2008-1330 [DOI] [PubMed] [Google Scholar]
- Butler, J. E., Sinkora M., Wertz N., Holtmeier W., and Lemke C. D.. . 2006. Development of the neonatal B and T cell repertoire in swine: implications for comparative and veterinary immunology. Vet. Res. 37:417–441. doi: 10.1051/vetres:2006009 [DOI] [PubMed] [Google Scholar]
- Campos, P. H., Labussière E., Hernández-García J., Dubois S., Renaudeau D., and Noblet J.. . 2014. Effects of ambient temperature on energy and nitrogen utilization in lipopolysaccharide-challenged growing pigs. J. Anim. Sci. 92:4909–4920. doi: 10.2527/jas.2014-8108 [DOI] [PubMed] [Google Scholar]
- Duan, X. D., Chen D. W., Zheng P., Tian G., Wang J. P., Mao X. B., Yu J., He J., Li B., Huang Z. Q., and Ao Z. G.. . 2016. Effects of dietary mannan oligosaccharide supplementation on performance and immune response of sows and their offspring. Anim. Feed Sci. Technol. 218:17–25. doi: 10.1016/j.anifeedsci.2016.05.002 [DOI] [Google Scholar]
- Fan, Y., and Pedersen O.. . 2021. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 19:55–71. doi: 10.1038/s41579-020-0433-9 [DOI] [PubMed] [Google Scholar]
- FASS. 2010. Guide for care and use of agricultural animals in research and teaching. 3rd ed. Champaign (IL): Federation of Animal Science Societies. [Google Scholar]
- Gutierrez, N. A., Kerr B. J., and Patience J. F.. . 2013. Effect of insoluble-low fermentable fiber from corn-ethanol distillation origin on energy, fiber, and amino acid digestibility, hindgut degradability of fiber, and growth performance of pigs. J. Anim. Sci. 91:5314–5325. doi: 10.2527/jas.2013-6328 [DOI] [PubMed] [Google Scholar]
- Hinkle, E. E. 2012. The effect of dam parity on progeny growth performance, passive immunity, and gastrointestinal microbiota [PhD dissertation]. Lincoln (NE): University of Nebraska. [Google Scholar]
- Holscher, H. D. 2017. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 8:172–184. doi: 10.1080/19490976.2017.1290756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaetzel, C. S. 2014. Cooperativity among secretory IgA, the polymeric immunoglobulin receptor, and the gut microbiota promotes host–microbial mutualism. Immunol. Lett. 162:10–21. doi: 10.1016/j.imlet.2014.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr, B. J., Weber T. E., and Ziemer C. J.. . 2015. Dietary marker effects on fecal microbial ecology, fecal VFA, nutrient digestibility coefficients, and growth performance in finishing pigs. J. Anim. Sci. 93:2183–2190. doi: 10.2527/jas.2014-8633 [DOI] [PubMed] [Google Scholar]
- Lee, J., Awji E., Lee S., Tassew D., Park Y., Park K., Kim M., Kim B., and Park S.. . 2012a. Effect of CJLP243 on the growth performance and cytokine response of weaning pigs challenged with enterotoxigenic Escherichia coli. J. Anim. Sci. 90:3709–3717. doi: 10.2527/jas.2011-4434 [DOI] [PubMed] [Google Scholar]
- Lee, J. H., Valeriano V. D., Shin Y. R., Chae J. P., Kim G. B., Ham J. S., Chun J., and Kang D. K.. . 2012b. Genome sequence of Lactobacillus mucosae LM1, isolated from piglet feces. J. Bacteriol. 194:4766. doi: 10.1128/JB.01011-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. H., Jiang X. R., Wang W. J., and Qiao J. Y.. . 2018. Effects of Lactobacillus acidophilus and zinc oxide on the growth performance, jejunal morphology and immune function of weaned piglet following an Escherichia coli K88 challenge. Ital. J. Anim. Sci. 17:114–120. doi: 10.1080/1828051X.2017.1344573 [DOI] [Google Scholar]
- Li, J., and Kim I. H.. . 2013. Effects of levan-type fructan supplementation on growth performance, digestibility, blood profile, fecal microbiota, and immune responses after lipopolysaccharide challenge in growing pigs. J. Anim. Sci. 91:5336–5343. doi: 10.2527/jas.2013-6665 [DOI] [PubMed] [Google Scholar]
- Li, Y., Xia S., Jiang X., Feng C., Gong S., Ma J., Fang Z., Yin J., and Yin Y.. . 2021. Gut microbiota and diarrhea: an updated review. Front. Cell. Infect. Microbiol. 11:1–8. doi: 10.3389/fcimb.2021.625210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y., Chen F., Odle J., Lin X., Jacobi S. K., Zhu H., Wu Z., and Hou Y.. . 2012. Fish oil enhances intestinal integrity and inhibits TLR4 and NOD2 signaling pathways in weaned pigs after LPS challenge. J. Nutr. 142:2017–2024. doi: 10.3945/jn.112.164947 [DOI] [PubMed] [Google Scholar]
- Louis, P., and Flint H. J.. . 2009. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 294:1–8. doi: 10.1111/j.1574-6968.2009.01514.x [DOI] [PubMed] [Google Scholar]
- Mao, J., Qi S., Cui Y., Dou X., Luo X. M., Liu J., Zhu T., Ma Y., and Wang H.. . 2020. Lactobacillus rhamnosus GG attenuates lipopolysaccharide-induced inflammation and barrier dysfunction by regulating MAPK/NF-κB signaling and modulating metabolome in the piglet intestine. J. Nutr. 150:1313–1323. doi: 10.1093/jn/nxaa009 [DOI] [PubMed] [Google Scholar]
- McDonnell, M., Bouwhuis M., Sweeney T., O’Shea C., and O’Doherty J.. . 2016. Effects of dietary supplementation of galactooligosaccharides and seaweed-derived polysaccharides on an experimental Salmonella Typhimurium challenge in pigs. J. Anim. Sci. 94(Suppl. 3):153–156. doi: 10.2527/jas.2015-9779 [DOI] [Google Scholar]
- Medzhitov, R., Preston-Hurlburt P., Kopp E., Stadlen A., Chen C., Ghosh S., and C. A.Janeway, Jr. 1998. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol. Cell 2:253–258. doi: 10.1016/s1097-2765(00)80136-7 [DOI] [PubMed] [Google Scholar]
- Miguel, J. C., Rodriguez-Zas S. L., and Pettigrew J. E.. . 2004. Efficacy of a mannan oligosaccharide (Bio-Mos®) for improving nursery pig performance. J. Swine Health Prod. 12:296–307. [Google Scholar]
- Nochta, I., Halas V., Tossenberger J., and Babinszky L.. . 2010. Effect of different levels of mannan‐oligosaccharide supplementation on the apparent ileal digestibility of nutrients, N‐balance and growth performance of weaned piglets. J. Anim. Physiol. Nutr. 94:747–756. doi: 10.1111/j.1439-0396.2009.00957.x [DOI] [PubMed] [Google Scholar]
- NRC. 2012. Nutrient requirements of swine. 11th ed. Washington D.C: National Academies Press. [Google Scholar]
- Ohland, C. L., and Macnaughton W. K.. . 2010. Probiotic bacteria and intestinal epithelial barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 298:G807–G819. doi: 10.1152/ajpgi.00243.2009 [DOI] [PubMed] [Google Scholar]
- Peng, L., Li Z. R., Green R. S., Holzman I. R., and Lin J.. . 2009. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 139:1619–1625. doi: 10.3945/jn.109.104638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluske, J. R., Williams I. H., and Aherne F. X.. . 1995. The neonatal pig - development and survival. In: Varley, M. A., editor. Nutrition of the neonatal pig. Wallingford UK: CAB International; p. 187–235. [Google Scholar]
- Rubio, L. A., Ruiz R., Peinado M. J., and Echavarri A.. . 2010. Morphology and enzymatic activity of the small intestinal mucosa of Iberian pigs as compared with a lean pig strain. J. Anim. Sci. 88:3590–3597. doi: 10.2527/jas.2010-3040 [DOI] [PubMed] [Google Scholar]
- Saminathan, M., Sieo C. C., Kalavathy R., Abdullah N., and Ho Y. W.. . 2011. Effect of prebiotic oligosaccharides on growth of Lactobacillus strains used as a probiotic for chickens. Afr. J. Microbiol. Res. 5:57–64. doi: 10.5897/AJMR10.700 [DOI] [Google Scholar]
- Sanchez, N. B., Carroll J. A., Broadway P. R., Bass B. E., and Frank J. W.. . 2019. Supplementation of a Lactobacillus acidophilus fermentation product can attenuate the acute phase response following a lipopolysaccharide challenge in weaned pigs. Animal 13:144–152. doi: 10.1017/S1751731118001222 [DOI] [PubMed] [Google Scholar]
- Soerensen, D. D., and Pedersen L. J.. . 2015. Infrared skin temperature measurements for monitoring health in pigs: a review. Acta Vet. Scand. 57:5. doi: 10.1186/s13028-015-0094-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Z., Li H., Li Y., and Qiao J.. . 2020. Lactobacillus salivarius, a potential probiotic to improve the health of LPS-challenged piglet intestine by alleviating inflammation as well as oxidative stress in a dose-dependent manner during weaning transition. Front. Vet. Sci. 7:547425. doi: 10.3389/fvets.2020.547425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Y., Zhou L., Fang L., Su Y., and Zhu W.. . 2015. Responses in colonic microbial community and gene expression of pigs to a long-term high resistant starch diet. Front. Microbiol. 6:877. doi: 10.3389/fmicb.2015.00877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titgemeyer, E. C., Bourquin L. D., G. C.Fahey, Jr., and Garleb K. A.. . 1991. Fermentability of various fiber sources by human fecal bacteria in vitro. Am. J. Clin. Nutr. 53:1418–1424. doi: 10.1093/ajcn/53.6.1418 [DOI] [PubMed] [Google Scholar]
- Tran, H., Bundy J. W., Li Y. S., Carney-Hinkle E. E., Miller P. S., and Burkey T. E.. . 2014. Effects of spray-dried porcine plasma on growth performance, immune response, total antioxidant capacity, and gut morphology of nursery pigs. J. Anim. Sci. 92:4494–4504. doi: 10.2527/jas.2014-7620 [DOI] [PubMed] [Google Scholar]
- Valeriano, V., Parungao-Balolong M., and Kang D.. . 2014. In vitro evaluation of the mucin‐adhesion ability and probiotic potential of Lactobacillus mucosae LM1. J. Appl. Microbiol. 117:485–497. doi: 10.1111/jam.12539 [DOI] [PubMed] [Google Scholar]
- Valpotić, H., Barić-Rafaj R., Mrljak V., Grabarević Ž., Samardžija M., Šperanda M., Žaja I. Ž., Đuričić D., Bach A., Harapin I., . et al. 2017. Influence of dietary mannan oligosaccharide and clinoptilolite on hematological, biochemical and gut histological parameters in weaned pigs. Period. Biol. 119. doi: 10.18054/pb.v119i1.4407 [DOI] [Google Scholar]
- Wang, S., Yao B., Gao H., Zang J., Tao S., Zhang S., Huang S., He B., and Wang J.. . 2019. Combined supplementation of Lactobacillus fermentum and Pediococcus acidilactici promoted growth performance, alleviated inflammation, and modulated intestinal microbiota in weaned pigs. BMC Vet. Res. 15:239. doi: 10.1186/s12917-019-1991-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, L. A., Newman M. C., Cromwell G. L., and Lindemann M. D.. . 2002. Brewers dried yeast as a source of mannan oligosaccharides for weanling pigs. J. Anim. Sci. 80:2619–2628. doi: 10.2527/2002.80102619x [DOI] [PubMed] [Google Scholar]
- Yoshida, M., Kobayashi K., Kuo T. T., Bry L., Glickman J. N., Claypool S. M., Kaser A., Nagaishi T., Higgins D. E., Mizoguchi E., . et al. 2006. Neonatal Fc receptor for IgG regulates mucosal immune responses to luminal bacteria. J. Clin. Invest. 116:2142–2151. doi: 10.1172/JCI27821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi, H., Wang L., Xiong Y., Wen X., Wang Z., Yang X., Gao K., and Jiang Z.. . 2018. Effects of Lactobacillus reuteri LR1 on the growth performance, intestinal morphology, and intestinal barrier function in weaned pigs. J. Anim. Sci. 96:2342–2351. doi: 10.1093/jas/sky129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, E., Chen D., Yu B., Huang Z., Mao X., Zheng P., Luo Y., Yin H., Yu J., Luo J., . et al. 2021. Manno-oligosaccharide attenuates inflammation and intestinal epithelium injury in weaned pigs upon enterotoxigenic Escherichia coli K88 challenge. Br. J. Nutr. 126:993–1002. doi: 10.1017/S0007114520004948 [DOI] [PubMed] [Google Scholar]
- Zeyner, A., and Boldt E.. . 2006. Effects of a probiotic Enterococcus faecium strain supplemented from birth to weaning on diarrhoea patterns and performance of piglets. J. Anim. Physiol. Anim. Nutr. (Berl). 90:25–31. doi: 10.1111/j.1439-0396.2005.00615.x [DOI] [PubMed] [Google Scholar]
- Zhang, L., Xu Y. Q., Liu H. Y., Lai T., Ma J. L., Wang J. F., and Zhu Y. H.. . 2010. Evaluation of Lactobacillus rhamnosus GG using an Escherichia coli K88 model of piglet diarrhoea: Effects on diarrhoea incidence, faecal microflora and immune responses. Vet. Microbiol. 141:142–148. doi: 10.1016/j.vetmic.2009.09.003 [DOI] [PubMed] [Google Scholar]
- Zhao, J., Harper A. F., Estienne M. J., K. E.Webb, Jr., McElroy A. P., and Denbow D. M.. . 2007. Growth performance and intestinal morphology responses in early weaned pigs to supplementation of antibiotic-free diets with an organic copper complex and spray-dried plasma protein in sanitary and nonsanitary environments. J. Anim. Sci. 85:1302–1310. doi: 10.2527/jas.2006-434 [DOI] [PubMed] [Google Scholar]
- Zhao, P. Y., Jung J. H., and Kim I. H.. . 2012. Effect of mannan oligosaccharides and fructan on growth performance, nutrient digestibility, blood profile, and diarrhea score in weanling pigs. J. Anim. Sci. 90:833–839. doi: 10.2527/jas.2011-3921 [DOI] [PubMed] [Google Scholar]
- Zhao, P. Y., Wang J. P., and Kim I. H.. . 2013. Evaluation of dietary fructan supplementation on growth performance, nutrient digestibility, meat quality, fecal microbial flora, and fecal noxious gas emission in finishing pigs. J. Anim. Sci. 91:5280–5286. doi: 10.2527/jas.2012-5393 [DOI] [PubMed] [Google Scholar]
- Zhou, H., Yu B., He J., Mao X., Zheng P., Yu J., Luo J., Luo Y., Yan H., and Chen D.. . 2020. The optimal combination of dietary starch, non-starch polysaccharides, and mannan-oligosaccharide increases the growth performance and improves butyrate-producing bacteria of weaned pigs. Animals 10:1745. doi: 10.3390/ani10101745 [DOI] [PMC free article] [PubMed] [Google Scholar]