Supplemental Digital Content is available in the text.
Keywords: adolescent, atherosclerosis, blood pressure, body mass index, carotid-femoral pulse wave velocity, obesity, young adult
Abstract
We examined the temporal longitudinal associations of carotid-femoral pulse wave velocity (cfPWV), a measure of arterial stiffness, and carotid intima-media thickness (cIMT) with the risk of overweight/obesity and elevated blood pressure (BP)/hypertension. We studied 3862 adolescents aged 17.7 years from the Avon Longitudinal Study of Parents and Children, followed-up for 7 years. cfPWV and cIMT were measured by ultrasound. Total and trunk fat mass and lean mass were assessed by dual-energy X-ray absorptiometry. Body mass index and BP were measured. Data were analyzed using logistic regression, linear mixed-effect, and cross-lagged structural equation models, with covariate adjustments. Among 1719 male and 2143 female participants, higher cfPWV at 17.7 years was associated with the risk of elevated systolic BP/hypertension (odds ratio, 1.20 [1.02–1.41]; P=0.026), elevated diastolic BP/hypertension (1.77 [1.32–2.38]; P<0.0001), body mass index-overweight/obesity (1.19 [1.01–1.41]; P=0.041), and trunk fat mass overweight/obesity (1.24 [1.03–1.49]; P=0.023) at 24.5 years. Higher cIMT at 17.7 years had no associations with obesity and elevated BP at follow-up. cfPWV progression was directly associated with 7-year increase in systolic BP (effect estimate 16 mm Hg [9–24]; P<0.0001) and diastolic BP (28 mm Hg [23–34]; P<0.0001). cIMT progression was directly associated with the 7-year increase of all adiposity measures and diastolic BP. In the temporal analysis, baseline cfPWV was directly associated with follow-up systolic and diastolic BP, however, baseline BP was unassociated with follow-up cfPWV. cfPWV but not cIMT was bidirectionally associated with adiposity. Obesity and hypertension prevention from adolescence may require developing novel approaches to mitigate arterial stiffness.
The global prevalence of obesity1 and hypertension2 in adolescence and young adulthood is on the rise. These twin risk factors of cardiovascular morbidity and mortality progress from childhood.1–6 Although obesity, elevated blood pressure (BP), and hypertension are well-established risk factors for arterial stiffness and carotid intima-media thickness (cIMT), surrogate markers of atherosclerotic cardiovascular diseases, a temporal or bidirectional relationship remains unexamined among adolescents and young adults.3–5,7–13
An American Heart Association’s scientific statement recommended that future studies investigate the natural history of arterial stiffness, obesity, and BP in relation to the rate at which arterial stiffness and BP increase with age.4 Similarly, another American Heart Association’s scientific statement just published recommended addressing the global burden of obesity hypertension through a life-course strategy of prevention and control, especially from a young age.13 From a public health perspective, it is important to determine whether decreasing arterial stiffness and cIMT from adolescence could independently reduce overweight/obesity and elevated BP/incident hypertension in early adulthood because studies in the age group are lacking.4,13 Therefore, we examined the temporal and bidirectional longitudinal associations of cfPWV and cIMT with total fat mass, trunk fat mass, lean mass, body mass index (BMI), and BP among adolescents and young adults, using data from the ALSPAC (Avon Longitudinal Study of Parents and Children) birth cohort, England, United Kingdom.
Methods
Data Availability Statement
The informed consent obtained from ALSPAC participants does not allow the data to be made freely available through any third-party maintained public repository. However, data used for this submission can be made available on request to the ALSPAC Executive. The ALSPAC data management plan describes in detail the policy regarding data sharing, which is through a system of managed open access. Full instructions for applying for data access can be found here: http://www.bristol.ac.uk/alspac/researchers/access/. Please note that the study website contains details of all the data that is available through a fully searchable data dictionary and variable search tool: (http://www.bristol.ac.uk/alspac/researchers/our-data/).
Study Cohort
Descriptions of the ALSPAC birth cohort have been published earlier14–16 and detailed in the Appendix in the Supplemental Material. Data were from the ALSPAC birth cohort, which investigates factors that influence childhood development and growth. Altogether, 14 541 pregnancies from women residing in Avon, southwestern England, United Kingdom, who had a total of 14 676 fetuses, were enrolled between April 1, 1991, and December 31, 1992. When the oldest children were ≈7 years of age, an attempt was made to bolster the initial sample with eligible cases who had failed to join the study originally resulting in 913 additional pregnancies. The total sample size for analyses using any data collected after 7 years of age is 15 454 pregnancies, resulting in 15 589 fetuses. Of these 14 901 were alive at 1 year of age. Regular clinic visits of the children commenced at 7 years of age and are still ongoing. For our analysis, we included participants who had both cfPWV and cIMT measurements at age 17.7 years (Figure S1 in the Supplemental Material). The demographic characteristics of excluded participants were similar to those included in this study (Table S1). Ethical approval for the study was obtained from the ALSPAC Ethics and Law Committee and the Local Research Ethics Committees and informed consent for the use of data was obtained from participants accordingly. Study data at 24.5 years were collected and managed using REDCap electronic data capture tools.17
All blood biochemical values were serially measured from fasting samples at 17.7 and 24.5 years, and body composition (total fat mass, trunk fat mass, and lean mass) was assessed using a dual-energy X-ray absorptiometry scanner.10–12 Details of the methods used for the measurement of weight, height, BP, fasting lipid, insulin, glucose levels, high-sensitivity C-reactive protein are contained in the Appendix in the Supplemental Material. At age 17.7 years, cfPWV was computed from pressure waveforms obtained using the Vicorder device (Skidmore Medical, Bristol, United Kingdom), whereas cIMT was assessed by ultrasound using a linear 12-MHz transducer (Vivid7, GE Medical, Chicago, Illinois) as earlier reported. At 24.5 years, cfPWV was measured, 5 minutes after resting in a semiprone position, using a Vicorder instrument (Skidmore Medical, Bristol, United Kingdom) with 2 BP measurement channels and 2 Velcro pressure sensor cuffs applied over each of the carotid and femoral arteries. The cfPWV measurement was repeated until three readings that were within 0.5 m/s of each other had been recorded. The right and left common carotid arteries at age 24.5 years were imaged using an ultrasound machine (CardioHealth Panasonic and a 13.5 MHz linear array broadband transducer; probe; center frequency 9.0 MHz). We calculated BMI by dividing weight by squared height. Participants at >75th percentile of total fat mass, trunk fat mass, or having >24.9 kg/m2 of BMI were classified as overweight and obese while those below this cut points were classified as normal weight.18 A high lean mass category included participants having >75th percentile of lean mass while those below this threshold were considered having normal lean mass.18 Participants were categorized as normotensive if BP was <120/80 mm Hg and elevated BP/hypertension when BP was >120/80 mm Hg.19 Missing data were handled with multiple imputations10,11,20,21 Questionnaire to assess smoking behavior were administered at the 17.7-year and 24.5-year clinic visits. At the 17.7-year clinic visit, participants were briefly asked about their personal and family (mother, father, and siblings) medical history, such as a history of hypertension, diabetes, high cholesterol, and vascular disease. Moderate to vigorous physical activity at age 15.5 years was assessed with ActiGraphTM accelerometer worn for 7 days,10 whereas at 24.5 years moderate to vigorous physical activity was assessed using ActiGraph GT3X+ accelerometer device worn for 4 consecutive days,22 ideally starting the day after the clinic visit (see Supplemental Material and Tables S2 through S10).
Statistical Analysis
Participants’ descriptive characteristics were summarized as means and SD, medians, and interquartile ranges, or frequencies and percentages. We explored sex differences using Independent t tests, Mann-Whitney U tests, or χ2 tests for normally distributed, skewed or dichotomous variables, respectively. We assessed the normality of variables and logarithmically or reciprocally transformed skewed variables before further analysis.
We investigated the separate longitudinal associations of cfPWV and cIMT (predictors) at 17.7 years with each of total fat mass, trunk fat mass, lean mass, BMI, systolic BP, and diastolic BP (outcomes) categories at 24.5 years using logistic regression (Supplemental Methods). We also examined the separate associations of the 7-year progression in cfPWV and cIMT with the longitudinal progression in each of the outcomes from ages 17.7 through 24.5 years using linear mixed-effect models for repeated measures. Analyses were adjusted for sex, age at 17.7 years, and covariates measured at 17.7 and 24.5 years, such as low-density lipoprotein cholesterol, insulin, triglyceride, high-sensitivity C-reactive protein, high-density lipoprotein cholesterol, heart rate, fasting blood glucose, diastolic or systolic BP, and fat mass or lean mass depending on the outcome, smoking status, family history of hypertension/diabetes/high cholesterol/vascular disease, and moderate to vigorous physical activity at 15.5 and 24.5 years.
Lastly, we used structural equation modeling with autoregressive cross-lagged design23 (detailed in the Supplemental Material) to examine the separate temporal associations of cfPWV and cIMT with outcomes, adjusting for covariates listed above. All covariates were selected based on previous studies.10–12,18,24 We examined sex interactions and presented sex-stratified results and cross-sectional analysis results in Table S11. For sensitivity analyses, we examined the quartile categories (high, moderate-high, moderate-low, and low) of cfPWV and cIMT progression with the increase in body composition and BP and presented the result in Table S12. We considered differences and associations with a 2-sided P<0.05 as statistically significant and made conclusions based on effect estimates and their 95% CI or SEs. Analyses involving 40% of a sample of 10 000 ALSPAC children at 0.8 statistical power, 0.05 alpha, and 2-sided P value would show a minimum detectable effect size of 0.049 SDs if they had relevant exposure for a normally distributed quantitative variable.25 All statistical analyses were performed using SPSS statistics software, Version 27.0 (IBM Corp, Armonk, NY), and structural equation modeling was conducted using IBM AMOS version 27.0.
Results
Study Population and Characteristics
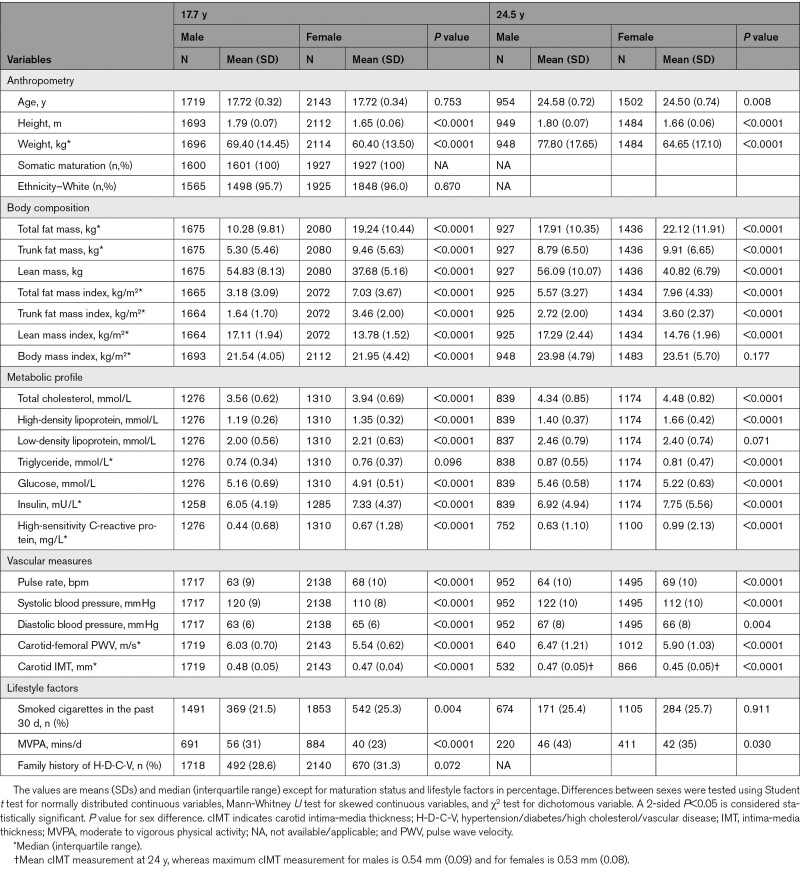
Altogether, 14 901 children in the ALSPAC birth cohort were alive at 1 year of age, of whom 5217 adolescents participated in the 17.7-year follow-up clinic visit, whereas 4026 young adults participated in the 24.5-year follow-up clinic visit (Figure S1). Only 3862 participants who had complete cfPWV and cIMT measurements at age 17.7 years were included in the study. The prevalence of overweight/obesity at 17.7 and 24.5 years was 20% and 38%, respectively. The prevalence of elevated systolic BP/hypertension at 17.7 and 24.5 years was 26% and 33%, respectively. Other characteristics of our study participants are shown in Table 1 and Tables S7 through S10.
Table 1.
Descriptive Characteristics of Cohort Participants
Longitudinal Associations of cfPWV and cIMT at 17.7 Years With Risk Categories of Obesity and Hypertension at Age 24.5 Years
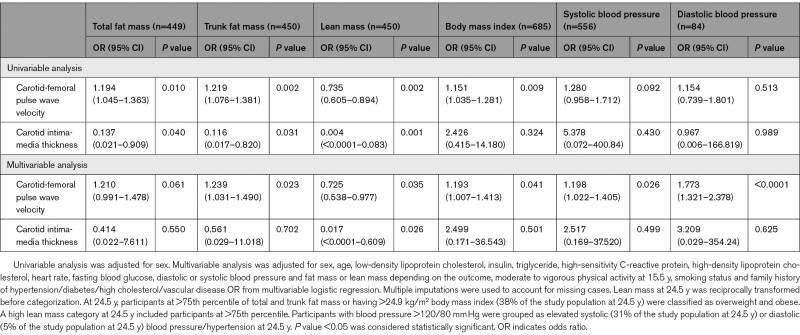
A higher cfPWV at 17.7 years predicted elevated systolic BP/hypertension (odds ratio [OR], 1.20 [95% CI, 1.02–1.41]; P=0.026), elevated diastolic BP/hypertension (OR, 1.77 [95% CI, 1.32–2.38]; P<0.0001), BMI-overweight/obesity (OR, 1.19 [95% CI, 1.01–1.41]; P=0.041), trunk fat mass-obesity (OR, 1.24 [95% CI, 1.03–1.49]; P=0.023), and decreased high lean mass (OR, 0.73 [95% CI, 0.54–0.98]; P=0.035) at age 24.5 years, after adjusting for cardiometabolic and lifestyle factors (Table 2). Among males, a higher cfPWV at 17.7 years predicted elevated systolic BP/hypertension (OR, 1.31 [95% CI, 1.02–1.70]; P=0.038) and elevated diastolic BP/hypertension (OR, 2.18 [95% CI, 1.49–3.19]; P<0.0001) at 24.5 years but not among females (OR, 1.09 [95% CI, 0.83–1.42]; P=0.542) and (OR, 1.40 [95% CI, 0.91–2.16]; P=0.122), respectively. There were no sex differences in the associations of higher cfPWV with obesity indices at 24.5 years. A higher cIMT at 17.7 years was only associated with the risk of decreased high lean mass at 24.5 years (OR, 0.02 [95% CI, 0.00–0.61]; P=0.026). There were no sex differences in the associations of higher cIMT at 17.7 years with higher obesity indices, body composition, and elevated BP at 24.5 years.
Table 2.
Carotid-Femoral Pulse Wave Velocity and Carotid Intima-Media Thickness at 17.7 y in Association With Risk Categories of Obesity and Hypertension at 24.5 y
Effect of cfPWV and cIMT Progression on Fat Mass, Lean Mass, and BP Progression From Ages 17.7 to 24.5 Years
A 7-year progression in cfPWV was directly associated with the 7-year increase in lean mass: (mean difference from baseline to follow-up 0.11 kg; [95% CI, 0.06–0.15; P<0.0001]), systolic BP: 16 mm Hg; (9–24; P<0.0001), and diastolic BP: 28 mm Hg; (23–34; P <0.0001) but negatively associated with trunk fat mass −0.19 kg (−0.35 to −0.02; P<0.0001) and BMI: −0.05 kg/m2; (−0.09 to −0.01; P=0.026), after adjustment for cardiometabolic and lifestyle factors (Table 3). The sex-stratified results were largely in consonance with the combined results (Table 3). Additional adjustments for light physical activity and sedentary time did not alter the results (data not shown). In the sensitivity analyses with the lowest quartile as the reference, the highest quartile of cfPWV progression from 17.7 through 24.5 years was directly associated with the 7-year increase in lean mass but negatively associated with the increase in total fat mass, trunk fat mass, and BMI (Table S12). In comparison with the lowest or reference category, the highest quartile of cfPWV progression was associated with the 7-year increase in systolic BP: 4 mm Hg; (3–5; P<0.0001) and diastolic BP: 3 mm Hg; (2–4; P<0.0001). The moderate-high cfPWV quartile was associated with the 7-year increase in systolic BP: 3 mm Hg; (1–4; P<0.0001) and diastolic BP: 2 mm Hg; (1–3; P<0.0001). The moderate-low cfPWV quartile was associated with the 7-year increase in systolic BP: 2 mm Hg; (1–3; P=0.001) and diastolic BP: 1 mm Hg (0.4–2; P=0.002; Table S12).
Table 3.
Longitudinal Progression in Arterial Stiffness and Carotid Intima-Media Thickness in Relation to Progression in Fat Mass, Lean Mass, and Blood Pressure From Age 17.7 to 24.5 y
A 7-year cIMT progression was directly associated with the 7-year increase in total fat mass, trunk fat mass, BMI, lean mass, and diastolic BP but negatively associated with systolic BP after adjustment for cardiometabolic and lifestyle factors (Table 3). Among males, there were no associations between cIMT progression and increase in lean mass and systolic BP. However, among females, cIMT progression was not associated with trunk fat mass and diastolic BP (Table 3). In the sensitivity analyses with the lowest quartile as the reference, the highest quartile of cIMT progression from 17.7 through 24.5 years was directly associated with the 7-year increase in lean mass and systolic BP but not associated with the increase in total fat mass, trunk fat mass, BMI, and diastolic BP (Table S12)
Cross-Lagged Temporal Relationships of cfPWV and cIMT With Fat Mass, Lean Mass, and BP
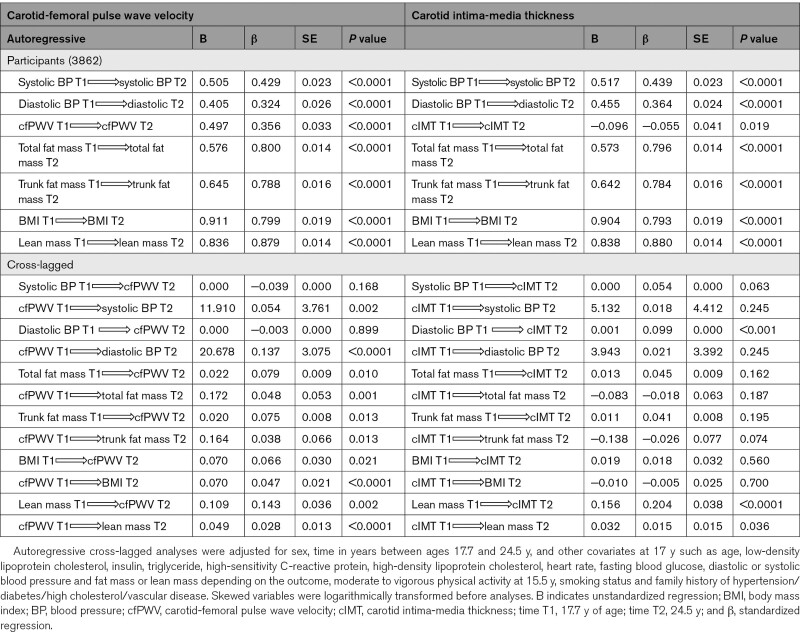
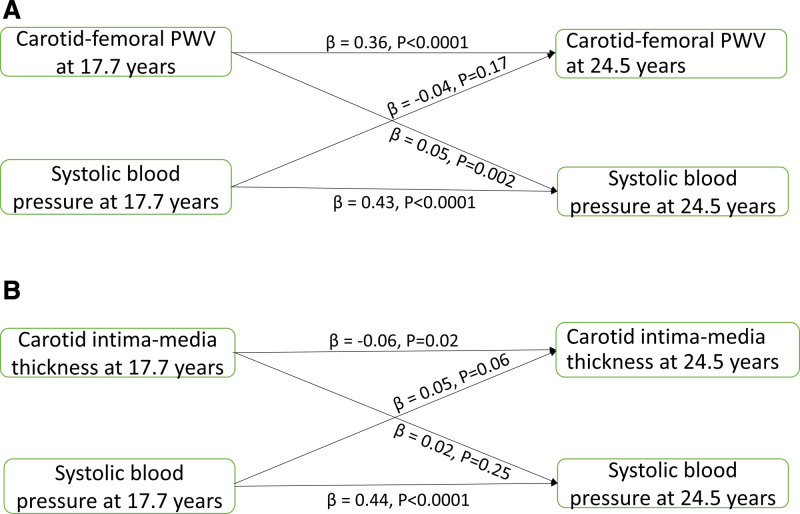
cfPWV, total fat mass, trunk fat mass, BMI, lean mass, systolic, and diastolic BP at 17.7 years were directly associated with their individual variables at 24.5 years; however, cIMT at 17.7 years was inversely associated with cIMT at 24.5 years (Table 4). We observed bidirectional associations between cfPWV and all measures of body composition, whereas cIMT had bidirectional associations with lean mass but had no relationships with adiposity indices (Figure 1A and 1B and Table 4). Higher cfPWV at 17.7 years was associated with high systolic and diastolic BP at 24.5 years, but neither systolic nor diastolic BP at 17.7 years was associated with cfPWV at 24.5 years (Figure 2A and Table 4). cIMT at 17.7 years was unrelated to systolic and diastolic BP at 24.5 years, whereas only diastolic BP at 17.7 years was associated with cIMT at 24.5 years (Figure 2B and Table 4).
Table 4.
Autoregressive Cross-Lagged Temporal Analyses of Carotid-Femoral Pulse Wave Velocity and Carotid Intima-Media Thickness With Fat Mass, Lean Mass, and Blood Pressure at 17.7 and 24.5 y of Age
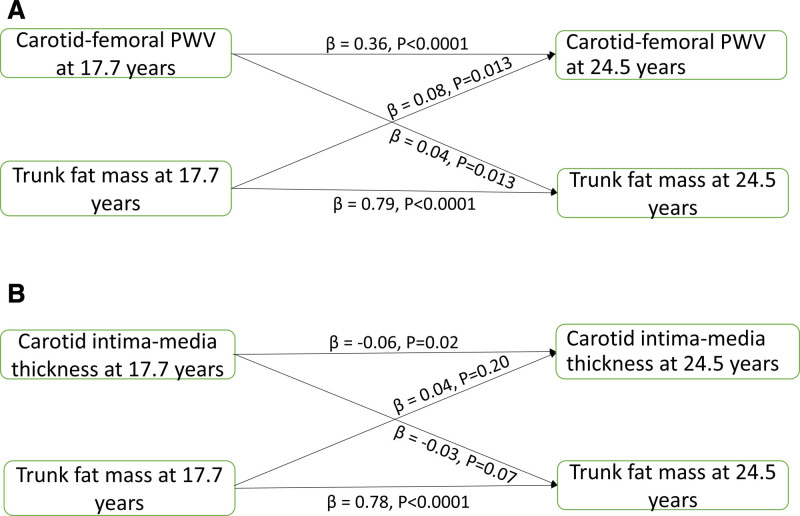
Figure 1.
Temporal causal relationships between arterial measures and body fat. Cross-lagged temporal analysis of arterial stiffness (A) and carotid intima-media thickness (B) progression with higher trunk fat mass among 3862 adolescents from the ALSPAC (Avon Longitudinal Study of Parents and Children) birth cohort. Analyses were adjusted for sex, time in years between ages 17.7 and 24.5 y, and other covariates at 17.7 y such as age, low-density lipoprotein cholesterol, insulin, triglyceride, high-sensitivity C-reactive protein, high-density lipoprotein cholesterol, heart rate, fasting blood glucose, systolic blood pressure, lean mass, moderate to vigorous physical activity at 15.5 y, smoking status and family history of hypertension/diabetes/high cholesterol/vascular disease. Skewed variables were logarithmically transformed before analyses. β, standardized regression. PWV indicates pulse wave velocity
Figure 2.
Temporal causal relationships between arterial measures and blood pressure. Cross-lagged temporal analysis of arterial stiffness (A) and carotid intima-media thickness (B) progression with higher systolic blood pressure in 3862 participants from the ALSPAC (Avon Longitudinal Study of Parents and Children) birth cohort. Analyses were adjusted for sex, time in years between ages 17.7 and 24.5 y, and other covariates at 17.7 y such as age, low-density lipoprotein cholesterol, insulin, triglyceride, high-sensitivity C-reactive protein, high-density lipoprotein cholesterol, heart rate, fasting blood glucose, fat mass, lean mass, moderate to vigorous physical activity at 15.5 y, smoking status and family history of hypertension/diabetes/high cholesterol/vascular disease. Skewed variables were logarithmically transformed before analyses. β indicates standardized regression; and PWV indicates pulse wave velocity.
Discussion
In a large longitudinal birth cohort, we showed for the first time that arterial stiffness, assessed by cfPWV, and cIMT during adolescence may be independent risk factors for obesity and incident hypertension in early adulthood. We thus conclude because first, we observed that cfPWV at 17.7 years independently predicted general and trunk obesity, decreased lean mass, and elevated BP/incident hypertension at 24.5 years. Second, we reported that a 7-year increase in cfPWV was directly associated with the 7-year increase in systolic BP and diastolic BP, whereas the longitudinal progression of cIMT was directly associated with the 7-year increase in total fat mass, trunk fat mass, lean mass, and diastolic BP. Lastly, using cross-lagged temporal structural equation models, cfPWV was bidirectionally associated with total fat mass, trunk fat mass, and lean mass but temporally preceded systolic and diastolic BP. cIMT was bidirectionally associated with lean mass only.
Arterial Stiffness with Obesity, Lean Mass, and Incident Hypertension
We and others have shown that cumulative exposure to total fat mass from childhood was associated with cfPWV in adolescence11 and in young adulthood.24 These previous findings11,24 and a meta-analysis3 involving 2237 children/adolescents (1281 with obesity, 956 healthy BMI controls) between 5 and 24 years of age emphasize a unidirectional relationship in which obesity is a strong and independent risk factor for arterial stiffness. However, to the best of our knowledge, it remains unknown whether arterial stiffness precedes (temporal relationship) incident obesity among a healthy young population. Our prospective analysis revealed that cfPWV at 17.7 years was independently associated with ≈20% increased risk of total and trunk fat mass overweight/obesity at age 24.5 years, although the 7-year increase in cfPWV was unrelated to the 7-year increase in total fat mass and trunk fat mass. Nonetheless, the cross-lagged findings showed that cfPWV had strong bidirectional associations with all measures of adiposity, suggesting that cfPWV may be a cause and a consequence of altered fat metabolism and deposit since increased arterial stiffness leads to high-flow low-resistance microvascular organ damage in the liver, pancreas, etc.5,8 By contrast, we observed that a higher cfPWV in late adolescence predicted ≈25% reduced lean mass in early adulthood. We have shown that higher lean mass from age 9 to 24 years may drive the increase in cfPWV from 17 to 24 years12 and in tandem with the cross-lagged findings, bidirectional relationships likely exist between cfPWV and muscle mass. In adults, arterial stiffness has been related to decreased lean mass via the mechanism of aging-induced chronic inflammation, hormonal dysregulation, impaired glucose metabolism, and other comorbidities.26
There is mounting evidence on the bidirectional relationship between arterial stiffness and BP and that arterial stiffness predates altered BP in the causal pathway.4,5,27 We have shown that BP is independently associated with higher cfPWV where a 15-year 1mm Hg rise in BP predicted a 0.013 m/s rise in cfPWV over a 7-year period.24 For the first time, we found in a young population, across cross-sectional, longitudinal, and cross-lagged analyses that arterial stiffness, measured with cfPWV, is a strong and consistent independent predictor of elevated BP/incident systolic and diastolic hypertension. We observed that a 1 m/s increase in cfPWV from ages 17.7 to 24.5 years was associated with a 4 mm Hg and 3 mm Hg increase in systolic and diastolic BP, respectively, over 7 years among participants in the highest quartile of cfPWV. Importantly, the cross-lagged findings suggest that arterial stiffness in late adolescence precedes the increase in systolic and diastolic BP in early adulthood. Baseline cfPWV did not predict elevated systolic and diastolic BP/hypertension at follow-up when controlled only for sex but was statistically significant after controlling for several potential confounders. This suggests that logistic regression analyses may be reported alongside path analysis or linear regression hierarchical models for the robustness of the results. Our findings, therefore, shed novel insights into the natural history of arterial stiffness and BP in relation to the rate at which arterial stiffness and BP increase with age, as identified as a research priority by the American Heart Association.4,13 Taken together, our finding buttress previous reports4,5,13 that arterial stiffness antedates and probably contribute to the pathogenesis of incident hypertension, independent of cardiometabolic, and lifestyle factors. The carotid-femoral artery has a thin collagenous adventitial layer, an elastin-ladened medial layer, and a thin intimal layer.4,5,8,27 The elastic media layer enables arterial distensibility during systole and blood flow augmentation during diastole. Elastic fiber degradation or an increased collagen deposit reduces the energy storing capacity of the elastic fiber and decreases collagen fiber undulation, thereby occasioning stiffened arteries.4,5,8,27 Arterial stiffness may lead to the remodeling of small resistance arteries which are implicated in the early phases of prehypertension. Damage to these arteries results in an elevated BP via wave reflections and propagation and may progressively aggravate into a vicious loop of increased arterial stiffness and more pressure wave reflections.4,5,8,27 Arterial stiffening increases characteristic impedance leading to higher wave reflections and pulse pressure propagation. This reflected wave returns earlier during ventricular contraction and increases late systolic BP.4,5,27
Carotid Intima-Media Thickness with Obesity, Lean Mass, and Incident Hypertension
Recent reports among adolescents10 and young adults12,24 seem to challenge the previous notion that obesity measured by BMI predicts cIMT progression in a healthy young population.9 These reports,10,12,24 in a large healthy young population, emphasized that dual-energy X-ray absorptiometry–measured total fat mass or trunk fat mass may not associate with cIMT progression during early life. Nonetheless, evidence on whether cIMT has a temporal relationship with body fat is lacking. In the longitudinal analysis, higher cIMT at 17.7 years of age had no relationship with all measures of adiposity at 24.5 years, both in the logistic regression and cross-lagged analyses. However, a 7-year progression in cIMT was independently and directly associated with the 7-year increase in total and central adiposity, especially in males. As expected, males had lower body fat content in contrast to females; however, there was a significant accumulation of fat mass among males during the 7-year growth. The median 7-year increase in total fat mass and trunk fat mass among males was 7.63 kg and 3.49 kg, respectively, whereas females had 2.88 kg of total fat mass and 0.45 kg of trunk fat mass increase. This 3- to 8-fold increase in fat mass among males relative to females may be a simultaneous increase not in the causal pathway of cIMT progression since cIMT increase did not precede the fat mass increase in the cross-lagged analyses.
We observed that the 7-year progression in cIMT was directly associated with the increase in lean mass from ages 17.7–24.5 years in both males and females, consistent with cross-sectional and cross-lagged analysis. In adolescents10 and young adults,12,24 cumulative high exposure to a dual-energy X-ray absorptiometry–measured lean mass was directly associated with cIMT10,24 and cIMT progression,24 but little is known regarding a reverse association (temporal association).28 We have now shown that a bidirectional relationship exists between cIMT and lean mass in a young healthy population, although the relationship is stronger when lean mass is the predictor. This temporal relationship may be due to a physiological carotid wall adaptation in association with skeletal muscle growth during maturation.10,24,26 It is important to note that higher cIMT at 17.7 years predicted a slight decrease in lean mass at 24.5 years. Considering the age and health status of our study population, an optimal cIMT may facilitate, decreased reactive oxidant species, rapid oxygen supply, increased myocyte proliferation, and increased production of anabolic hormones.9,26,28
cIMT measured at 17.7 years was unrelated to systolic or diastolic BP at 24.5 years either via logistic regression or cross-lagged analyses. However, a 7-year cIMT progression was independently and directly associated with the 7-year increase in diastolic BP, particularly among males. Although cIMT maximally increased by 0.06 mm between ages 17.7–24.5 years, among males, it explained ≈18% of the increase in diastolic BP during the observation period. This simultaneous rise in cIMT and intravascular pressure may suggest a compensatory nonatherosclerotic adaptation occurring between lumen diameter and cIMT via the maintenance of local wall shear stress.29
Strengths and Limitations of the Study
The ALSPAC dataset based on a large birth cohort provides an extensive array of gold-standard and repeated measures of variables during adolescence and young adulthood which allowed us to construct cross-lagged structural equation models to examine temporal and bidirectional associations. We could not adjust for diet as the variable was unavailable at 17.7- and 24.5-year follow-up clinic visits, nonetheless, participants’ body composition and metabolic state which we controlled for may reflect their diets. The absolute time in moderate to vigorous physical activity was a relatively small proportion of the day, although within the expected range.13,22 Therefore, we additionally controlled for sedentary time and light physical activity but the findings remained unchanged. We cannot exclude unmeasured or residual confounders, for instance, adiponectin has been associated with arterial stiffness progression.30 Although we could not control for the effect of adiponectin because the variable was measured only in childhood, we controlled for high-sensitivity C-reactive protein, another inflammatory marker associated with arterial stiffness progression.5 There were no regional differences in our study population as all were from the Avon area of England and were mostly White participants; therefore, we are unable to generalize our findings to other populations with different ethnicities. Lastly, the observational nature of our study may not establish causality; however, a temporal longitudinal path in the associations of cfPWV and cIMT with obesity and hypertension is a critical step in the causal pathway.
Perspectives
Adolescence arterial stiffness independently predicted trunk fat overweight/obesity, elevated BP/incident hypertension, and a decreased lean mass in early adulthood. Arterial stiffness appears to precede the increase in systolic and diastolic BP but the relationship with adiposity may be more complex and bidirectional. Increased cIMT at 17.7 years was associated with decreased lean mass at 24.5 years. A 7-year arterial stiffness progression, that is, persistently high cfPWV from 17.7 through 24.5 years, was strongly associated with the increase in systolic and diastolic BP, whereas cIMT progression was associated with the increase in all measures of adiposity at the end of the observation period. Reducing arterial stiffness and cIMT from adolescence may be effective in preventing elevated BP/hypertension and overweight/obesity risks in early adulthood. Nonetheless, future temporal longitudinal investigations of cfPWV and cIMT in mediating obesity and hypertension risk are warranted in a large multiethnic adolescent population.
Article Information
Acknowledgments
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC (Avon Longitudinal Study of Parents and Children) team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. A.O. Agbaje contributed to concept and design. A.O. Agbaje participated in drafting of the article. All authors contributed to acquisition, analysis, or interpretation of data and critical revision of the article for important intellectual content. A.O. Agbaje participated in statistical analysis. A.O. Agbaje obtained funding. A.R. Barker and T.-P. Tuomainen participated in supervision.
Sources of Funding
The UK Medical Research Council and Wellcome (Grant ref: 217065/Z/19/Z; 076467/Z/05/Z) and the University of Bristol provide core support for ALSPAC (Avon Longitudinal Study of Parents and Children). The British Heart Foundation grant (CS/15/6/31468) funded blood pressure, carotid intima-media thickness, carotid-femoral pulse wave velocity, and Actigraph activity monitoring device measurement at 24 years. The Medical Research Council grant (MR/M006727/1) supported smoking data collection. Comprehensive list of grants funding is available on the ALSPAC website (http://www.bristol.ac.uk/alspac/external/documents/grant-acknowledgements.pdf); This publication is the work of the authors, and A.O. Agbaje, A.R. Barker, and T.-P. Tuomainen will serve as guarantors for the contents of this paper. This research (A.O. Agbaje) was specifically funded by the Doctoral Program in Clinical Research, Institute of Public Health and Clinical Nutrition, Faculty of Health Sciences, University of Eastern Finland; the Jenny and Antti Wihuri Foundation (Grant no: 00180006); and the North Savo regional and central Finnish Cultural Foundation (Grants no: 65191835 and 00200150). The University of Eastern Finland funded open access publishing. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; and decision to submit the article for publication.
Disclosures
None.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- ALSPAC
- Avon Longitudinal Study of Parents and Children
- BMI
- body mass index
- BP
- blood pressure
- cfPWV
- carotid-femoral pulse wave velocity
- cIMT
- carotid intima-media thickness
- OR
- odds ratio
For Sources of Funding and Disclosures, see page 168.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.121.18449.
Hypertension is available at www.ahajournals.org/journal/hyp
Contributor Information
Alan R. Barker, Email: A.R.Barker@exeter.ac.uk.
Tomi-Pekka Tuomainen, Email: tomi-pekka.tuomainen@uef.fi.
Novelty and Significance
What Is New?
The temporal longitudinal associations of repeatedly measured arterial stiffness (carotid-femoral pulse wave velocity) and carotid intima-media thickness with the risk of elevated blood pressure/hypertension and overweight/obesity risk among adolescents and young adults are unknown.
What Is Relevant?
Adolescent arterial stiffness may be a precursor of hypertension and other metabolic risk factors in young adulthood rather than a consequence.
Summary
Persistently high carotid-femoral pulse wave velocity from adolescence through young adulthood was strongly associated with elevated blood pressure/hypertension and obesity, suggesting that obesity and hypertension prevention from adolescence may require developing novel approaches to mitigate arterial stiffness.
References
- 1.NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390:2627–2642. doi: 10.1016/S0140-6736(17)32129-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song P, Zhang Y, Yu J, Zha M, Zhu Y, Rahimi K, Rudan I. Global prevalence of hypertension in children: a systematic review and meta-analysis. JAMA Pediatr. 2019;173:1154–1163. doi: 10.1001/jamapediatrics.2019.3310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cote AT, Phillips AA, Harris KC, Sandor GG, Panagiotopoulos C, Devlin AM. Obesity and arterial stiffness in children: systematic review and meta-analysis. Arterioscler Thromb Vasc Biol. 2015;35:1038–1044. doi: 10.1161/ATVBAHA.114.305062 [DOI] [PubMed] [Google Scholar]
- 4.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, et al. ; American Heart Association Council on Hypertension. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension. 2015;66:698–722. doi: 10.1161/HYP.0000000000000033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boutouyrie P, Chowienczyk P, Humphrey JD, Mitchell GF. Arterial stiffness and cardiovascular risk in hypertension. Circ Res. 2021;128:864–886. doi: 10.1161/CIRCRESAHA.121.318061 [DOI] [PubMed] [Google Scholar]
- 6.Juonala M, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, Srinivasan SR, Daniels SR, Davis PH, Chen W, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011;365:1876–1885. doi: 10.1056/NEJMoa1010112 [DOI] [PubMed] [Google Scholar]
- 7.Raitakari OT, Juonala M, Kähönen M, Taittonen L, Laitinen T, Mäki-Torkko N, Järvisalo MJ, Uhari M, Jokinen E, Rönnemaa T, et al. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA. 2003;290:2277–2283. doi: 10.1001/jama.290.17.2277 [DOI] [PubMed] [Google Scholar]
- 8.Chirinos JA, Segers P, Hughes T, Townsend R. Large-artery stiffness in health and disease: JACC state-of-the-art review. J Am Coll Cardiol. 2019;74:1237–1263. doi: 10.1016/j.jacc.2019.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doyon A, Kracht D, Bayazit AK, Deveci M, Duzova A, Krmar RT, Litwin M, Niemirska A, Oguz B, Schmidt BM, et al. ; 4C Study Consortium. Carotid artery intima-media thickness and distensibility in children and adolescents: reference values and role of body dimensions. Hypertension. 2013;62:550–556. doi: 10.1161/HYPERTENSIONAHA.113.01297 [DOI] [PubMed] [Google Scholar]
- 10.Chiesa ST, Charakida M, Georgiopoulos G, Dangardt F, Wade KH, Rapala A, Bhowruth DJ, Nguyen HC, Muthurangu V, Shroff R, et al. Determinants of intima-media thickness in the young: the ALSPAC study. JACC Cardiovasc Imaging. 2021;14:468–478. doi: 10.1016/j.jcmg.2019.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dangardt F, Charakida M, Georgiopoulos G, Chiesa ST, Rapala A, Wade KH, Hughes AD, Timpson NJ, Pateras K, Finer N, et al. Association between fat mass through adolescence and arterial stiffness: a population-based study from The Avon Longitudinal Study of Parents and Children. Lancet Child Adolesc Health. 2019;3:474–481. doi: 10.1016/S2352-4642(19)30105-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agbaje AO, Barker AR, Tuomainen T-P. A 15-year cumulative high exposure to lean mass and blood pressure but not fat mass predicts the 7-year change in carotid-femoral pulse wave velocity and carotid intima-media thickness: the ALSPAC study. Circulation. 2021;143:A080–A080. doi: 10.1161/circ.143.suppl_1.080 [Google Scholar]
- 13.Hall ME, Cohen JB, Ard JD, Egan BM, Hall JE, Lavie CJ, Ma J, Ndumele CE, Schauer PR, Shimbo D; American Heart Association Council on Hypertension; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Lifestyle and Cardiometabolic Health; and Stroke Council. Weight-loss strategies for prevention and treatment of hypertension: a scientific statement from the American Heart Association. Hypertension. 2021;78:e38–e50. doi: 10.1161/HYP.0000000000000202 [DOI] [PubMed] [Google Scholar]
- 14.Boyd A, Golding J, Macleod J, Lawlor DA, Fraser A, Henderson J, Molloy L, Ness A, Ring S, Davey Smith G. Cohort profile: the ‘children of the 90s’–the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol. 2013;42:111–127. doi: 10.1093/ije/dys064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fraser A, Macdonald-Wallis C, Tilling K, Boyd A, Golding J, Davey Smith G, Henderson J, Macleod J, Molloy L, Ness A, et al. cohort Profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol. 2013;42:97–110. doi: 10.1093/ije/dys066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Northstone K, Lewcock M, Groom A, Boyd A, Macleod J, Timpson N, Wells N. The Avon Longitudinal Study of Parents and Children (ALSPAC): an update on the enrolled sample of index children in 2019. Wellcome Open Res. 2019;4:51. doi: 10.12688/wellcomeopenres.15132.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, et al. ; REDCap Consortium. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ness AR, Leary SD, Mattocks C, Blair SN, Reilly JJ, Wells J, Ingle S, Tilling K, Smith GD, Riddoch C. Objectively measured physical activity and fat mass in a large cohort of children. PLoS Med. 2007;4:e97. doi: 10.1371/journal.pmed.0040097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bakris G, Ali W, Parati G. ACC/AHA versus ESC/ESH on hypertension guidelines: JACC guideline comparison. J Am Coll Cardiol. 2019;73:3018–3026. doi: 10.1016/j.jacc.2019.03.507 [DOI] [PubMed] [Google Scholar]
- 20.Rubin DB. An overview of multiple imputation. Proc Surv Res methods Sect Am Stat Assoc. 1988;16:79–84. [Google Scholar]
- 21.Mackinnon A. The use and reporting of multiple imputation in medical research - a review. J Intern Med. 2010;268:586–593. doi: 10.1111/j.1365-2796.2010.02274.x [DOI] [PubMed] [Google Scholar]
- 22.Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–188. doi: 10.1249/mss.0b013e31815a51b3 [DOI] [PubMed] [Google Scholar]
- 23.Browne MW, Cudeck R. Bollen KA, Long JS, eds. Alternative ways of assessing model fit. Testing Structural Equation Models. 1993. Sage; 136–162. [Google Scholar]
- 24.Agbaje AO, Barker AR, Tuomainen T-P. Longitudinal associations of fat mass, lean mass, body mass index and blood pressure from childhood through young adulthood with carotid-femoral pulse wave velocity and carotid intima-media thickness at age 24.5 years. J Am Coll Cardiol. 2021;77(18_suppl_1):1490. doi:10.1016/S0735-1097(21)02848-5 [Google Scholar]
- 25.Golding G, Pembrey P, Jones J. ALSPAC - The Avon Longitudinal Study of Parents and Children I. Study methodology. Paediatr Perinat Epidemiol. 2001;15:74–87.. doi: 10.1046/j.1365-3016.2001.00325.x [DOI] [PubMed] [Google Scholar]
- 26.Tap L, Kirkham FA, Mattace-Raso F, Joly L, Rajkumar C, Benetos A. Unraveling the links underlying arterial stiffness, bone demineralization, and muscle loss. Hypertension. 2020;76:629–639. doi: 10.1161/HYPERTENSIONAHA.120.15184 [DOI] [PubMed] [Google Scholar]
- 27.Safar ME, Asmar R, Benetos A, Blacher J, Boutouyrie P, Lacolley P, Laurent S, London G, Pannier B, Protogerou A, et al. ; French Study Group on Arterial Stiffness. Interaction between hypertension and arterial stiffness. Hypertension. 2018;72:796–805. doi: 10.1161/HYPERTENSIONAHA.118.11212 [DOI] [PubMed] [Google Scholar]
- 28.Dvoretskiy S, Lieblein-Boff JC, Jonnalagadda S, Atherton PJ, Phillips BE, Pereira SL. Exploring the association between vascular dysfunction and skeletal muscle mass, strength and function in healthy adults: a systematic review. Nutrients. 2020;12:E715. doi: 10.3390/nu12030715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bots ML, Hofman A, Grobbee DE. Increased common carotid intima-media thickness. Adaptive response or a reflection of atherosclerosis? Findings from the Rotterdam Study. Stroke. 1997;28:2442–2447. doi: 10.1161/01.str.28.12.2442 [DOI] [PubMed] [Google Scholar]
- 30.Youn JC, Kim C, Park S, Lee SH, Kang SM, Choi D, Son NH, Shin DJ, Jang Y. Adiponectin and progression of arterial stiffness in hypertensive patients. Int J Cardiol. 2013;163:316–319. doi: 10.1016/j.ijcard.2011.06.061 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The informed consent obtained from ALSPAC participants does not allow the data to be made freely available through any third-party maintained public repository. However, data used for this submission can be made available on request to the ALSPAC Executive. The ALSPAC data management plan describes in detail the policy regarding data sharing, which is through a system of managed open access. Full instructions for applying for data access can be found here: http://www.bristol.ac.uk/alspac/researchers/access/. Please note that the study website contains details of all the data that is available through a fully searchable data dictionary and variable search tool: (http://www.bristol.ac.uk/alspac/researchers/our-data/).