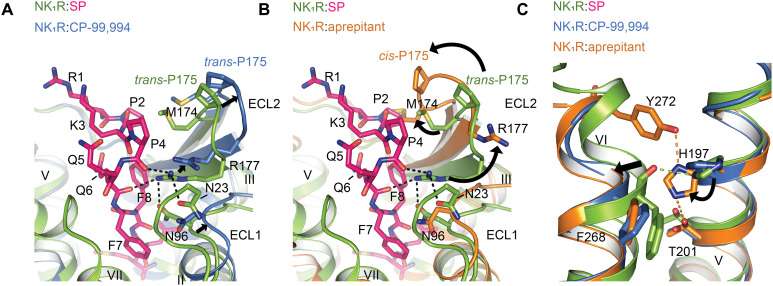
Fig. 5. The insurmountable antagonist-bound NK1R state prohibits SP binding.
(A) Superposition of agonist-bound NK1R:SP:Gq (green) with the surmountable antagonist CP-99,994–bound NK1R (PDB ID: 6HLL) (blue) reveals a slightly outward-moved but similar ECL2 conformation for the antagonist conformation. Note the small movement of R177 ECL2. (B) Superposition of agonist-bound NK1R:SP:Gq with the insurmountable antagonist aprepitant-bound NK1R (PDB ID: 6HLO) reveals an inward shifted and twisted ECL2 in the insurmountable conformation that clashes at M174ECL2 with P4 of the endogenous agonist and also poses R177ECL2 away from its central position in the polar network at the extracellular receptor surface. (C) Superposition of agonist-bound NK1R:SP:Gq, CP-99,994- and aprepitant-bound NK1R showing the hydrogen bonds (indicated as orange, dotted lines) that lock helices V and VI in the insurmountable conformation. The rotamer orientation of H1975.39 in the insurmountable conformation prevents the necessary inward movement of helix VI and the formation of a new hydrogen bond between H1975.39 with the backbone carbonyl oxygen of F2686.55 (indicated as green, dotted line) in the active state.