Fig. 1. Structural diversity of cobamides.
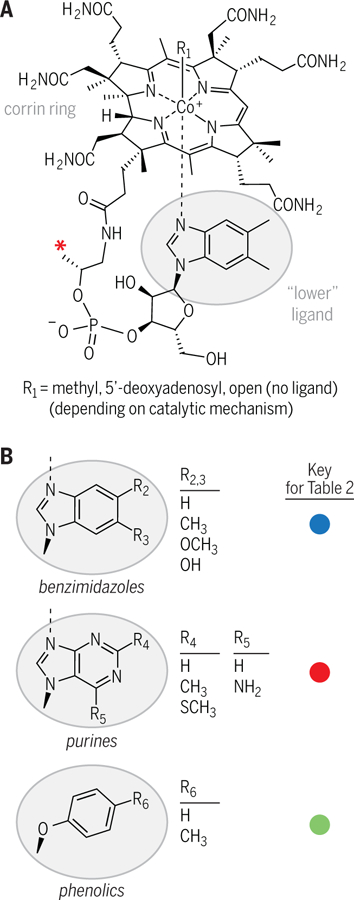
(A) The chemical structure of cobalamin (B12), the most well-known cobamide. Cobamides are characterized by a corrin ring (a contracted porphyrin, similar to the macrocycles of heme and chlorophyll) which houses a cobalt ion. Exchangeable upper ligands (R1) give cobamides versatile chemical reactivity (6). Lower ligands differ between cobalamin and other cobamides. The red asterisk indicates a methyl group that is absent in norcobamides (35, 36). (B) Three lower ligand structural classes are shown, which have multiple variable regions (R2 to R6).
