SUMMARY
Objective:
Infantile spasms may evolve into persistent epilepsies including Lennox-Gastaut syndrome. We compared adult epilepsy outcomes in models of infantile spasms due to structural etiology (multiple-hit model) or focal cortical inflammation and determined the antiepileptogenic effects of pulse-rapamycin, previously shown to stop spasms in multiple-hit rats.
Methods:
Spasms were induced in 3-days old male rats via right intracerebral doxorubicin/lipopolysaccharide (multiple-hit model) infusions. Controls and sham rats were used. Separate multiple-hit rats received pulse-rapamycin or vehicle intraperitoneally between postnatal days 4–6. In adulthood, video-EEG scoring for seizures and sleep, and histology were done blinded to treatment.
Results:
Motor-type seizures developed in 66.7% multiple-hit rats, usually from sleep, but were reduced in the pulse-rapamycin treated group (20%, p=0.043 vs multiple-hit) and rare in other groups (0–9.1%, p<0.05 vs multiple-hit). Spike-and-wave bursts had slower frequency in multiple-hit rats (5.4–5.8Hz) than the other groups (7.6–8.3Hz) (p<0.05); pulse rapamycin had no effect on the hourly spike-and-wave burst rates in adulthood. Rapamycin, however, reduced the time spent in slow-wave-sleep (17.2%), which was increased in multiple-hit rats (71.6%, p=0.003). Sham rats spent more time in wakefulness (43.7%) compared to controls (30.6%, p=0.043). Multiple-hit rats, with or without rapamycin treatment, had right more than left cortico-hippocampal, basal ganglia lesions. There was no macroscopic pathology in the other groups.
Significance:
Structural cortico-hippocampal/basal ganglia lesions increase the risk for post-infantile spasms epilepsy, Lennox-Gastaut syndrome features, and sleep dysregulation. Pulse-rapamycin treatment for infantile spasms has antiepileptogenic effects, despite the structural lesions, and decreases time spent in slow wave sleep.
Keywords: epilepsy, West syndrome, Lennox-Gastaut syndrome, sleep, mTOR
INTRODUCTION
Infantile spasms (IS) are age-specific seizures characterized by clusters of axial epileptic flexion and extension spasms in infancy1–7. In ~24% there is a genetic or genetic-structural etiology whereas in a third the cause is unknown7–11. In ~60% of affected infants, IS are associated with pre-existing structural or metabolic brain pathologies, and have worse prognosis than IS of unknown etiology, including persistence of intellectual and other neurological deficits as well as epilepsies that are often drug-resistant4, 5, 7, 12. These epilepsies are most commonly associated with focal or multifocal seizures5. Less frequently, IS evolve into Lennox-Gastaut syndrome (LGS), with the characteristic slow spike-wave discharges (slow-SWDs) and tonic seizures during sleep13. In IS of unknown etiology, early cessation of spasms may partially improve neurodevelopmental outcomes14 but has no effect on subsequent epilepsy5. There are currently no known antiepileptogenic therapies for IS.
Several chronic models of IS exist, including genetic or acquired15–18. We have extensively used the multiple-hit rat model of IS to screen for new therapeutics for IS. The multiple-hit model of IS due to structural lesions [DLP model; PN3: right intracerebroventricular doxorubicin and right intracortical lipopolysaccharide, PN5 intraperitoneal (i.p.) p-chlorophenylalanine]17, 19–24 manifests spasms that are refractory to adrenocorticotropic hormone (ACTH) and partially and transiently responsive to vigabatrin17. Among the various treatments that have been tested in the DLP model19–23, a pulse 3 day-treatment protocol with the mTOR (mechanistic target of rapamycin) inhibitor rapamycin, given at PN4–6 after spasms onset, stopped spasms and improved visuospatial learning, suggestive of partial disease modification, despite the existing structural lesions19.
Here, we determined the adult epilepsy outcomes in the DLP model as well as the antiepileptogenic potential of pulse rapamycin given at PN4–6, after spasms onset, in DLP rats, in regards to both motor-type seizures and spike-wave discharges (SWDs). To investigate whether rats with IS develop LGS features and if pulse rapamycin modifies them, we also compared the SWD characteristics and sleep-wake periods across models and as a function of rapamycin treatment.
MATERIAL AND METHODS
Animals
The male offspring of timed pregnant Sprague-Dawley rats (Taconic farms, Inc., Hudson, NY, USA) were used in the experiments. Rats were maintained at constant temperature (21–23°C) and humidity (40–60%) in a 12h dark/12h light cycle with free access to pellet food and water in our animal facility, which is accredited by the American Association for the Accreditation of Laboratory Animal Care. Rat pups were weaned at PN21 and were housed with 1–2 same-sex littermates till the electrode placement day. The rats were individually housed after the implantation of the EEG electrodes. Experiments were approved by the Institutional Animal Care and Use Committee of the Albert Einstein College of Medicine and were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Model induction and pulse rapamycin treatment
Induction protocols, timeline, treatments, sample sizes and spasm phenotypes are described in Table 1A. PN3 surgeries were done under isoflurane anesthesia (4.5% induction, 1.5% maintenance, in O2). To document the expression of spasms, all rats except controls underwent intermittent video monitoring between PN4–20, with 1–2 daily sessions, each lasting for 2h as described previously17, 19–23. Other male DLP rats received either rapamycin (6, 3, 3 mg/kg/day on PN4, PN5, PN6 respectively, n=10; LC Laboratories, Woburn MA, USA) or equal volumes of vehicle (DLP-ETP, n=5) i.p.. ETP vehicle consisted of 4% ethanol, 5% Tween-80 and 5% polyethylene glycol 400 (Fisher Scientific, Pittsburgh PA, USA)19. Because the incidence of adult seizures was similar in DLP and DLP-ETP rats, these two groups have been grouped together.
Table 1.
Experimental treatment groups and terminology of EEG patterns
| Table 1A. Experimental treatment groups | ||||
|---|---|---|---|---|
| Treatment group | Age of treatment | Treatments | Spasms (PN4-13) |
N |
| DLP (all) | Combination of DLP and DLP-ETP rats (see next cells) | Yes | 12 male | |
| - DLP | PN3 | Doxorubicin (5μg/2.5μl in saline, right i.c.v.) LPS (3μg/1.5μl in saline, R.i.c.) |
Yes | 7 male |
| PN5 | p-chlorophenylalanine (200mg/kg, i.p.) | |||
| - DLP-ETP | PN3 and PN5 | Same as DLP | Yes |
5 male |
| PN4–6 | ETP vehicle given i.p. once daily, equal volume as rapamycin treatment | |||
| DLP-RAP633 | PN3 and PN5 | Same as DLP induction | Yes | 10 male |
| PN4–6 | Rapamycin given i.p. once daily, 6mg/kg on PN4, 3mg/kg on PN5 and PN6 | |||
| Sham | PN3 | Saline (1.5μl, right intraparietal) | No | 8 male |
| Controls | No early life handling or surgery | Not monitored (*) | 11 male | |
| Table 1B. EEG pattern terminology | |
|---|---|
| Motor-type seizure1 | Stereotypical patterns that disrupt the background, with evolution in field, frequencies, or amplitude, consist of a mixture of rhythmic activities and/or spikes or polyspikes, > 10 sec, that were associated with or were similar to seizures with motor manifestations |
| Early seizure onset patterns | First changes of EEG from the baseline during a seizure |
| Late seizure onset patterns | Follow the early seizure onset patterns and precede the generalized rhythmic spikes and seizure activity |
| Low voltage fast (LVF) | Low amplitude beta (>13 Hz) activity that was visibly different than the background |
| High amplitude alpha bursts (HA a bursts) | Alpha bursts with higher amplitude and field and more sharply contoured morphology than the rat’s sleep spindles |
| Epileptiform discharges (EDs) | Spikes or polyspikes or rhythmic spike-and-wave discharges (SWDs) |
| Ictal onset attenuation (IA) | Attenuation of background voltage (i.e., <50% of prior background), loss of background frequency variability |
| Rhythmic θ/α pattern | Rhythmic evolving theta/alpha activities, +/− rhythmic EDs, that lead to a seizure. |
| Spike-and-slow wave discharge (SWDs) bursts | Stereotypical bursts of runs of rhythmic SWDs >1sec that were distinct from the background (>3x background voltage), and had relatively sudden onset and offset. |
| Wake-sleep state scoring | |
| - Awake state (AW) | Low voltage EEG activity accompanied by exploratory behavior and appearance of theta or alpha activity26, 27. |
| - Slow wave sleep (SWS) | Slow frequencies in the EEG background, including high amplitude delta waves, and frontocentral sleep spindles (7–16 Hz) accompanied by immobility. |
| - “Other” state | REM sleep and passive wakefulness were scored as “other” due to the lack of EMG and eye movement recordings. |
| Sleep spindles | 7–16 Hz frontocentral spindle-like activity seen in SWS |
Controls were not monitored (PN4–13) to avoid stress or handling. Prior experiments had not demonstrated clusters of spasms in video-monitored naïve control rats at this age.
Abbreviations: ETP: vehicle for rapamycin, i.e. 4% ethanol, 5% Tween-80 and 5% polyethylene glycol 400; i.c.v.: intracerebroventricularly; i.p.: intraperitoneally; LPS: lipopolysaccharide; PN: postnatal day; R.i.c.: right intracortically.
Seizure scoring scale: The motor behavioral correlates of seizures were classified using the Racine scale as modified by Pinel & Rovner (stage 1–8)25: stage 1, facial automatisms (chewing, jaw clonus); stage 2, head nodding; stage 3, unilateral forelimb clonus; stage 4, body jerks, bilateral forelimb clonus and rearing; stage 5, stage 4, loss of righting reflex; stage 6, repeated stage 5 seizures; stage 7, running, jumping and/or rolling; stage 8, stage 7 with periods of tonus.
EEG electrode implantation and long-term video-EEG monitoring
Stainless steel epidural cortical screw electrodes (Plastics One, Roanoke, VA) were implanted stereotactically in adulthood (Table 2), under ketamine (70 mg/kg, i.p.) and xylazine (6 mg/kg, i.p.) anesthesia at frontal (anterior to bregma 1.5 mm, lateral ± 2 mm), central (posterior to bregma 2.8 mm, lateral ± 2 mm) and occipital (posterior to bregma: 6 mm, lateral: ± 4 mm) regions bilaterally. The electrodes were connected to a plastic pedestal (Plastics One, Roanoke, VA) which was fixed to the skull using dental cement (Henry Schein, Dublin OH, USA).
Table 2.
Group comparisons on video-EEG monitoring, motor-type seizures and SWD bursts
| Feature | DLP | DLP-RAP633 | Sham | Control |
|---|---|---|---|---|
| Rats / group | 12 | 10 | 8 | 11 |
| Age at onset of monitoring (PN day) | 78.8 ± 8.8 | 69.9 ± 6.8 | 122.4±37.5 | 93.4±36.8 |
| Age at end of monitoring (PN day) | 177.9 ± 79.4 | 169.5±69.3 | 239.8±50.2 | 247.4±32.7 |
| Rat-days of monitoring | 35.9±25.2 | 46.6±26.7 | 23±11.2 | 65.9±35.3 |
| Motor-type seizures | ||||
| Rats with seizures | 8/12 (66.7%) | 2/10 (20%) | 0/10 (0%) | 1/11 (9.1%) |
| Total seizures (n) | 26 total; 25 with EEG |
12 | 0 | 1 |
| Mean age at first detection (PN day) | 110.3 ± 30.3 (range 68–137) |
140.5 ± 64.3 (range 95–186) |
N/A | 173 |
| Incidence of seizures | 66.7% (8/12 rats) |
20% (2/10 rats) |
0% (0/8) |
9.1% (1/11) |
| State dependence | n of seizures (%) | n of seizures (%) | n of seizures (%) | |
| SWS | 20/25 (80%) | 8/12 (66.7%) | N/A | N/A (no pre-ictal) |
| AW | 5/25 (20%)# | 4/12(33.3%) | N/A | N/A (no pre-ictal) |
| Mean electrographic seizure duration (sec) (Min-max) | 83 ± 101 (range 17 – 481 sec) |
157 ± 112 (range 62–360 sec) |
N/A | End of seizure only |
| Stage 1 * | 1/18 (5.6%) | 0/12 (0%) | N/A | 0/1 (0%) |
| Stage 2 | 1/18 (5.6%) | 0/12 (0%) | N/A | 1/1 (100%) |
| Stage 3 | 7/18 (38.9%) | 1/12 (8.3%) | N/A | 0/1 (0%) |
| Stage 4 | 3/18 (16.7%) | 1/12 (8.3%) | N/A | 0/1 (0%) |
| Stage 5 | 1/18 (5.6%) | 0/12 (0%) | N/A | 0/1 (0%) |
| Stage 6 | 4/18 (22.2%) | 8/12 (66.7%) | N/A | 0/1 (0%) |
| Stage 7 | 0/18 (0%) | 2/12 (16.7%) | N/A | 0/1 (0%) |
| Stage 8 | 1/18 (5.6%) | 0 (0%) | N/A | 0/1 (0%) |
| NCSE | 1/18 (5.6%) | 0 (0%) | N/A | 0/1 (0%) |
| No video | 8/26 (30.8%) | 0 (0%) | N/A | 0/1 (0%) |
| First change in: | ||||
| EEG | 10/17 (58.8%) | 12/12 (100%) | N/A | N/A |
| Behavior | 2/17 (11.8%) | 0/12 (0%) | N/A | N/A |
| Both | 5/17 (29.4%) | 0/12 (0%) | N/A | N/A |
| Unclear/no video | 8/26 (30.8%) | 0 | N/A | 0/1 (0%) |
| Unclear/no EEG | 1/26 (3.8%) | 0 | N/A | 1/1 (100%) |
| SWD bursts | ||||
| Rats with SWD bursts | 4/12 (33.3%) | 5/10 (50%) | 5/8 (62.5%) | 7/11 (63.6%) |
| Mean age at first detection (PN day) | 79 ± 7.5 | 107 ± 57.1 | 144.8 ± 22.8 | 146.6 ± 24.4 |
| Main SWD frequency (Hz) | 5.4 ± 0.8 | 5.8 ± 1.1 | 8.3 ± 0.3 | 7.6 ± 0.5 |
AW: awake; N/A: Not applicable; NCSE: nonconvulsive status epilepticus; PN day: postnatal day; SWS: slow wave sleep.
Seizures in AW state occurred in two rats with NCSE or very epileptic background.
Seizure semiology in this stage 1 seizure was also described as being “jerky, ataxic, restless”
Video-EEG monitoring commenced one week after surgery using the XLTEK system (Natus Medical Incorporated, San Carlos, CA, USA) and a Panasonic V500M camcorder (1920×1080/60p; Panasonic, Tokyo, Japan) synchronized to EEG and focused on up to 5 rat monitoring cages. The EEG signals were amplified without filtering and digitized at 200Hz. Rats were monitored in 24h sessions, 2 or 3 days per week. Video was turned on between 9am-6pm.
Video-EEG and sleep-wake scoring
Video-EEG scoring and spectral analysis were done blinded to treatment and confirmed by board certified neurophysiologists. Definitions of EEG patterns are described in Table 1B. The behavioral seizure correlates were classified using the Racine scale as modified by Pinel & Rovner (stage 1–8)25. SWD bursts were analyzed for their main frequency (Hz), focality, and rate of occurrence (SWD bursts/hr) in 24 h periods. Slow-wave-sleep (SWS) and awake (AW) states were analyzed during the light period from PN133-PN167 EEG recordings, to minimize the age effect on sleep/AW durations according to accepted criteria26, 27.
The spectral characteristics were analyzed by computing the power spectra using the Fast Fourier Transform (FFT) (MATLAB 7.6, MathWorks, Natick, USA). FFT was performed on 2 sec artifact-free epochs, tapered with Hamming window, and a frequency resolution of 0.5 Hz. The EEG data recorded from right and left central electrodes using the common reference were analyzed. To compare the frequency characteristics of SWDs among the five groups, 10 SWD bursts were selected per animal (PN135-PN200), time-locked to the onset of the first spike of the first SWD, and the power-spectra of the first 2 sec segments were averaged.
Histology
Rats were anesthetized with pentobarbital (100mg/kg i.p.) and transcardially perfused with cold saline and 10% neutral buffered formalin (Sigma-Aldrich, St Louis, MO), formalin-post-fixed and stored at −80° C until cut in 40 μm coronal brain sections (Microm International, Walldorf, Germany) and thionin-stained, as previously published17, 19.
Statistics
Fisher’s exact test was used to compare incidence of the seizures and SWDs among groups. Wilcoxon test was used to compare mean hourly SWD burst rates per rat across groups. Linear mixed model with interaction term analysis was performed to compare the changes in SWD bursts/hr rates as a function of age and group. P<0.05 were considered statistically significant. JMP Pro 16 and SAS9.4 (SAS Institute Inc., Cary, NC, U.S.A.) were used for statistical analyses. Means and standard deviations (SD) are presented.
RESULTS
Motor-type seizures in adulthood
The group comparisons of EEG and behavioral correlates of motor-type seizures are described in Table 2 and depicted in Fig 1, Fig 2, and Supplementary Fig 1. Spontaneous motor-type seizures were observed in 8/12 (66.7%) DLP rats, in 1/11 (9.1%) of controls and in 0/8 (0%) sham rats (p<0.05).
Fig 1. Motor-type seizures and SWD bursts in adulthood.
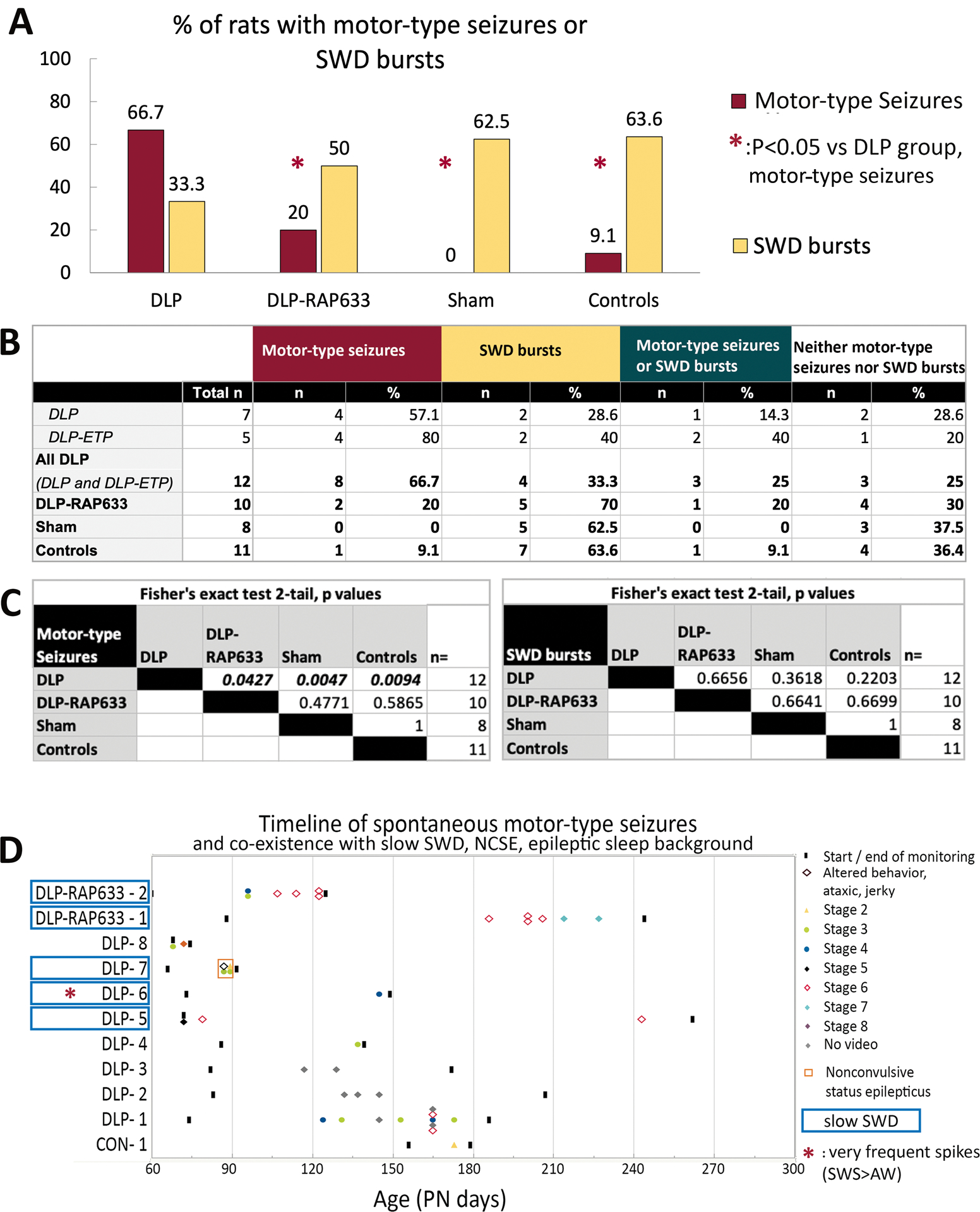
Panel A. DLP rats have a significantly higher incidence of motor-type seizures in adulthood than each of the other groups. Pulse rapamycin (DAP-RAP633) given on PN4–6 prevents the expression of motor-type seizures in adulthood suggesting an antiepileptogenic effect. SWD bursts are seen in all groups.
Panel B. Summary of incidence of motor-type seizures, SWD bursts in adult rats across the various studied groups.
Panel C. The matrices present the p values of the across-groups comparisons in motor-type seizure or SWD bursts incidences, using Fisher’s exact test.
Panel D. Timeline of spontaneous motor-type seizures and co-existence of motor-type seizures with slow-SWD burst phenotype (rats in cyan boxes), nonconvulsive status epilepticus (NCSE, red square frame) and very frequent spikes in SWS vs AW interictally (red asterisk). Only rats with spontaneous motor-type seizures are included in this panel. Three DLP rats and 2 DLP-RAP633 rats with motor-type seizures had slow-SWD bursts. DLP-7 rat with both slow-SWD and motor-type seizures had NCSE and eventually expired. DLP-6 rat with slow-SWD and motor-type seizures also had chronically epileptic background with bilateral spikes, mostly frontal, almost continuous in SWS, throughout the monitoring period.
Fig 2. Electrographic patterns during interictal slow wave sleep (SWS) (A), motor-type seizure onsets (B) and motor-type seizure patterns (C) in DLP adult rats.
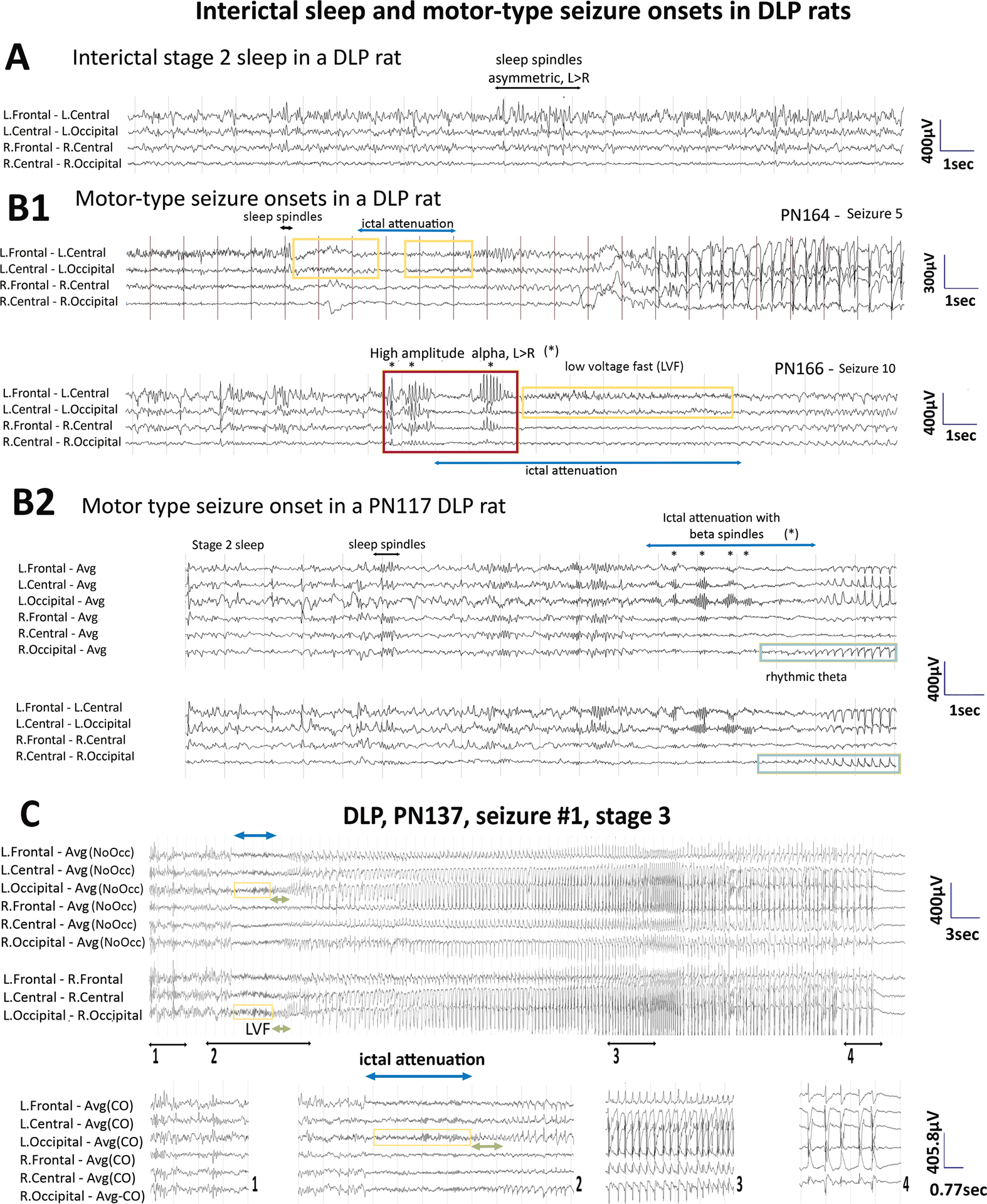
Panel A: Interictal SWS EEG of a PN164 DLP rat shows asymmetric background with more prominent sleep spindles at the left frontocentral region (black horizontal arrow).
Panel B: Onsets of motor-type seizures captured in two DLP rats. (B1) Seizure onsets from PN164 and PN166 are from the rat from panel A. Asymmetric sleep spindles in preictal SWS (black horizontal arrow) precede seizure onset in seizure 5 (PN164). The yellow boxes indicate the low voltage fast (LVF) at the onsets of seizure 5 and 10 and the cyan lines indicated ictal attenuations. Seizure 10 of the same rat starts with high amplitude alpha bursts (*, red box) with higher amplitude and sharper contour compared to the usual sleep spindles, more prominent at the left than the right hemisphere, maximal frontally. (B2) Seizure onset of a PN117 DLP rat shows pre-ictal sleep spindles in SWS. Ictal attenuation (cyan line) and left occipital beta spindles (*) emerge followed by rhythmic theta (green boxes) at the right occipital region that evolves into the seizure pattern.
Panel C: EEG of the first detected motor-type seizure (stage 3) of a PN137 adult DLP rat. The pre-ictal EEG shows asymmetric sleep spindle (maximal left frontocentral) (segment 1). Ictal attenuation at seizure onset (segment 2) with left occipital LVF leading into rhythmic theta which spreads to bilateral occipital regions evolving into a seizure pattern bilaterally (segment 3) which terminates with postictal attenuation (segment 4). EEG is presented with either average montage excluding occipital leads or transverse montage.
Sampling rate: 200Hz. Low frequency filter (LFF): 1Hz, high frequency filter (HFF): 70Hz. Avg: Average reference; Avg (NoOcc): average reference with occipital leads excluded; L: left, R: right.
DLP rats:
A total of 26 DLP seizures were captured. Eight events had unclear video and 1 was witnessed (stage 4) but no EEG was available. All seizures captured in video-EEG were electroclinical and the semiology (stage 1–8) is described in Table 2 and Fig 1. A cluster of 3 seizures within 10 hours was captured in one DLP rat. In the majority of the electroclinical seizures captured with video-EEG, the first change was observed in the EEG (58.8% of events) only or in both EEG and behavior (29.5%) (Fig 1, Table 2 and Supplementary Table 1). The two seizures where altered behaviors (jerky, ataxic, restless rat) were noted prior to the electrographic seizure onset occurred in a rat with nonconvulsive status epilepticus (NCSE) which eventually expired.
Most (80%) DLP motor-type seizures occurred in SWS, except for 2 rats that had seizures in AW in the context of either NCSE or a chronically epileptiform EEG background with almost continuous spikes in SWS more than in AW.
Among the 25 DLP seizures with EEG, focal onsets were seen in 13/25 (52%) of DLP seizures and these were predominantly left (12/13). Left hemispheric predominance was also seen in 76.9% of seizures with bilateral onsets. Bilateral EEG involvement was eventually present in all. The early and late EEG patterns at seizure onset (Table 1 for definitions) included rhythmic θ/α pattern (88%), focal or lateralized low voltage fast (LVF) (72%), epileptiform discharges (EDs) [72%], diffuse ictal onset attenuation (IA) for 4.1 ± 4.1 sec (76%), and left posterior beta spindles during IA (8%) (Supplementary Table 1, Fig 2).
Voltage attenuation occurred as the first change in EEG background prior to seizure development in 24% of seizures, all emerging from SWS. We therefore cannot exclude that it may represent in part a change of state. As a result, determination of focality in events starting with voltage attenuation was done based on the earliest other focal EEG change. Features that indicate that attenuation could also be an ictal feature include: (a) its emergence as late ictal onset patterns (52% or seizures), (b) rare appearance during seizures from AW (20% of seizures), even though IA was more common in seizures from sleep (90%, p=0.006). IA was not associated with any behavioral change in 14/19 seizure onsets, sudden awakening occurred in 4/19 IA and sudden movement in 1/19.
HA α bursts were more prominent at the left frontocentral area, were seen in 28% of DLP seizures (2/8 rats), all of which emerged from sleep.
Postictal diffuse attenuation occurred in 88% of motor-type seizures and postictal slowing was seen in the rest.
Sham and control rats:
We did not capture motor-type seizures in sham rats. The end of a stage 2 seizure was captured in a control PN173 rat after handling during connection to the EEG acquisition system. The EEG showed generalized rhythmic polyspikes (4–5/sec) slowing into a 1–2/sec rhythmicity and postictal voltage suppression.
Antiepileptogenic effects of pulse rapamycin in DLP rats
DLP rats that received pulse rapamycin on PN4–6 (DLP-RAP633) had significantly lower incidence of motor-type seizures (2/10 rats, 20%) than DLP rats (p=0.043, Fisher’s exact test) suggesting an antiepileptogenic effect. Seizures were either focal (usually left dominant) or bilateral (left dominant when lateralized) at onset with secondary generalization and 66.7% of them emerged from sleep. Seizure onset patterns were similar to DLP (Table 3) except that no HA α bursts were present. All seizures from AW in DLP-RAP633 were isolated events.
SWD bursts
SWD bursts were observed in all 4 groups without significant inter-group comparisons (Table 2, Fig 1 and Fig 3). Because many rats manifested SWD bursts around the first day of the recording, earlier age of onset could not be excluded and group comparisons were not done. Once present, SWD bursts occurred throughout the records except for 3/4 DLP, 3/5 DLP-RAP633, and 1/7 controls which had infrequent and brief SWD bursts at the last day of monitoring. SWD bursts occurred in both AW and SWS states.
Fig 3. SWD bursts in adult control, DLP, DLP-RAP633, and Sham rats using epidural recordings.
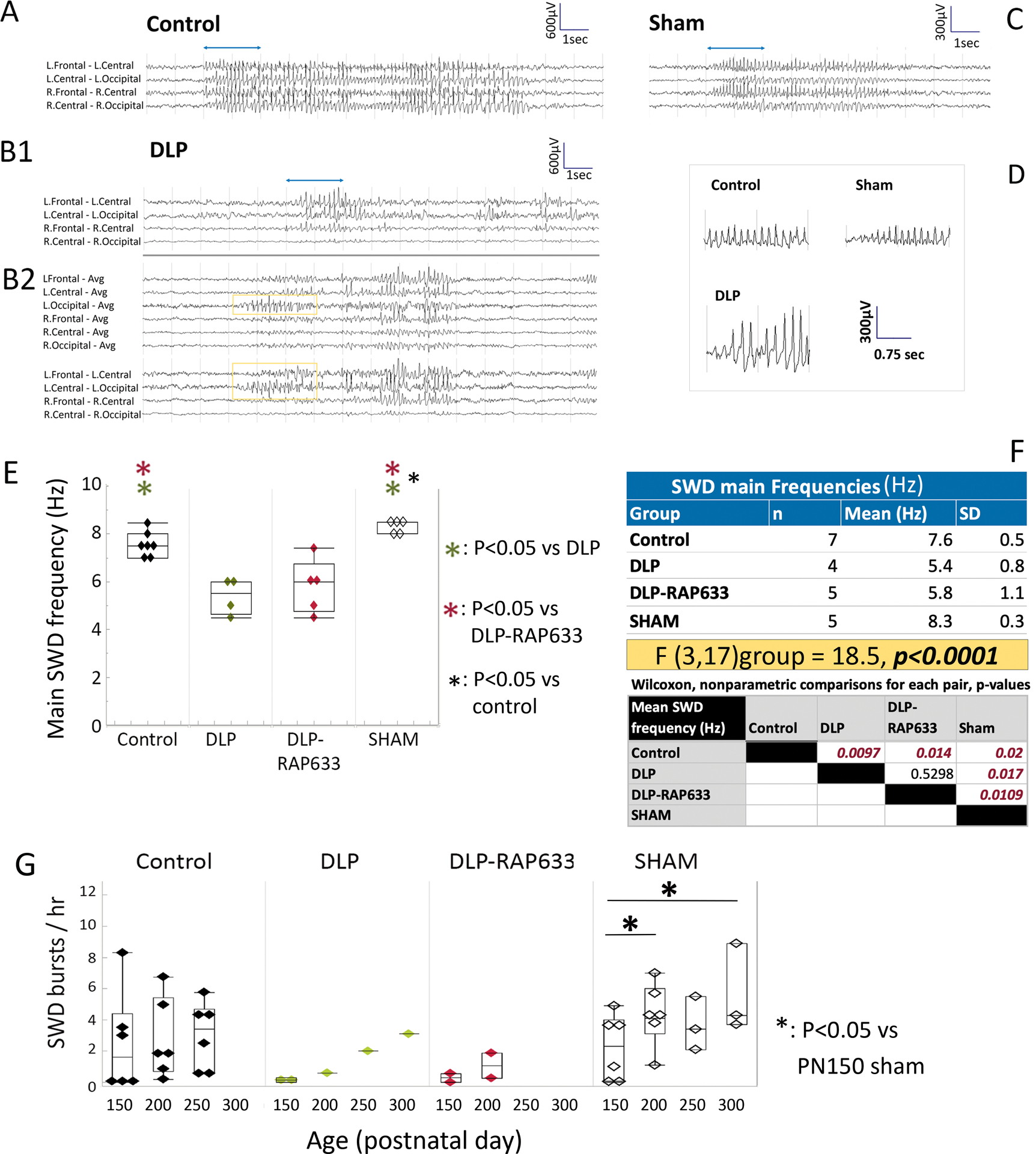
Panel A. Example of a SWD burst in a control rat. Bipolar montages demonstrate bilateral onsets maximal frontally.
Panel B1 and B2. Example of focal SWD burst in a DLP rat, maximal at the left frontal region (B1). Panel B2 shows an atypical SWD with a hypersynchronous lead with rhythmic left occipital SWDs (yellow box) evolving into a SWD.
Panel C. Example of a SWD burst in a SHAM operated rat.
Panel D. SWD waveforms from each panel are shown with an expanded time scale (first 2 sec of bursts in panels A-D, shown with the blue lines in panels A, B1, C).
Panels E-F. Main frequencies of SWD bursts in adult rats. Spectral characteristics of SWD bursts were done using the first 2 seconds of the bursts in the EEG tracings from the right and left central electrodes, referenced to a common reference. Comparisons of the mean SWD main frequency values of each rat indicated a significant group effect [F(3,17)group = 18.5, p<0.0001, mixed model analysis] (panel F). DLP and DLP-RAP633 have slower main SWD frequencies than sham or control rats (p<0.05, hence they are called here as “slow-SWD”. Sham rats also had faster SWD main frequencies compared to controls. No significant difference in SWD main frequencies between right and left central electrode recordings were found in the various groups.
Panel G. Rates of SWD bursts per hour in adult rats across different age groups (PN150, PN200, PN250, PN300) and groups. Comparison of the mean SWD burst rates (SWD bursts/hr) of each rat using Wilcoxon test did not yield significant group effects. Linear mixed model analysis of hourly rates of SWD bursts as a function of age, for each group separately, using repeated measures, indicated significant age-related increase for the sham group only [PN150 vs PN200, p=0.043; PN150 vs PN300, p=0.009], although DLP and DLP-RAP633 groups had small sample sizes.
During wakefulness, behavioral correlates of SWD bursts consisted of freezing or behavioral arrest during the electrographic SWD burst followed by head/body-jerk or sudden return to normal activity. Brief SWD bursts (< 3sec) had no clear behavioral correlate.
In sham, and control rats, SWD bursts were bilateral with occasional asymmetries at onset (Fig 3). When present, SWD bursts in DLP and DLP-RAP633 rats were asymmetric and more prominent at the left hemisphere. Atypical SWD bursts were seen in one DLP rat with a hypersynchronous focal onset of rhythmic SWD from the left occipital region leading into a SWD burst (Fig 3 B2).
Group effect was significant on the main frequency of SWD bursts [Fgroup(3,17)=18.5, p<0.001] (Fig 3, panels E and F). DLP and DLP-RAP633 had slower main frequencies (5.4–5.8Hz range of mean frequencies, p<0.05) than the other 2 groups (7.6–8.3Hz range of mean frequencies), which had similar SWD main frequencies as the models of classical absence (7–8Hz)28. As a result, SWD bursts in DLP or DLP-RAP633 are here referred as “slow-SWD” bursts.
Comparison of the mean SWD burst rates (SWD bursts/hr) of each rat using Wilcoxon test did not yield significant group effects. Because age-related increase in SWD bursts has been reported in other studies, we compared the hourly rates of long SWD bursts (≥3sec) across groups, using 24h EEGs and linear mixed model analysis, in PN156 (151–161), PN205 (201–209), and PN254 (249–259] rats. Linear mixed model analysis of hourly rates of SWD bursts across age groups, for each group, using repeated measures, indicated significant age-related increase for the sham group only [PN150 vs PN200, p=0.043; PN150 vs PN300, p=0.009]. Very few DLP and DLP-RAP633 rats had SWD bursts between PN150–300, which hindered meaningful statistics.
EEG background and LGS features
All adult DLP and DLP-RAP633 rats had interictal spikes at the left hemisphere (frontal, occipital or central) and 6/12 DLP and 6/10 DLP-RAP633 rats also had spikes at the right hemisphere. No spikes or sharp waveforms were observed in control, sham rats with no seizure or SWD bursts. The background was typically asymmetric in DLP or DLP-RAP633 rats with left hemispheric attenuation of background activities and sleep spindles (Fig 2). The background was symmetric in the other groups.
LGS features in DLP rats included the emergence of motor-type seizures from SWS in most DLP and DLP-RAP633 rats, and their occasional coexistence with slow-SWD bursts and/or NCSE and very epileptiform interictal EEG particularly in sleep (Fig 1D).
Sleep-Awake periods
Scoring of SWS, AW and “other” (REM vs calm wakefulness) periods in PN133–167 rats (Fig 4) revealed significantly more SWS and reduced AW periods in DLP rats than every other group. DLP-RAP633 rats spent more time in AW and less time in SWS than any other group. Sham rats also spent more time in AW than controls.
Fig 4. Sleep-wake periods and histological brain findings in adult rats.
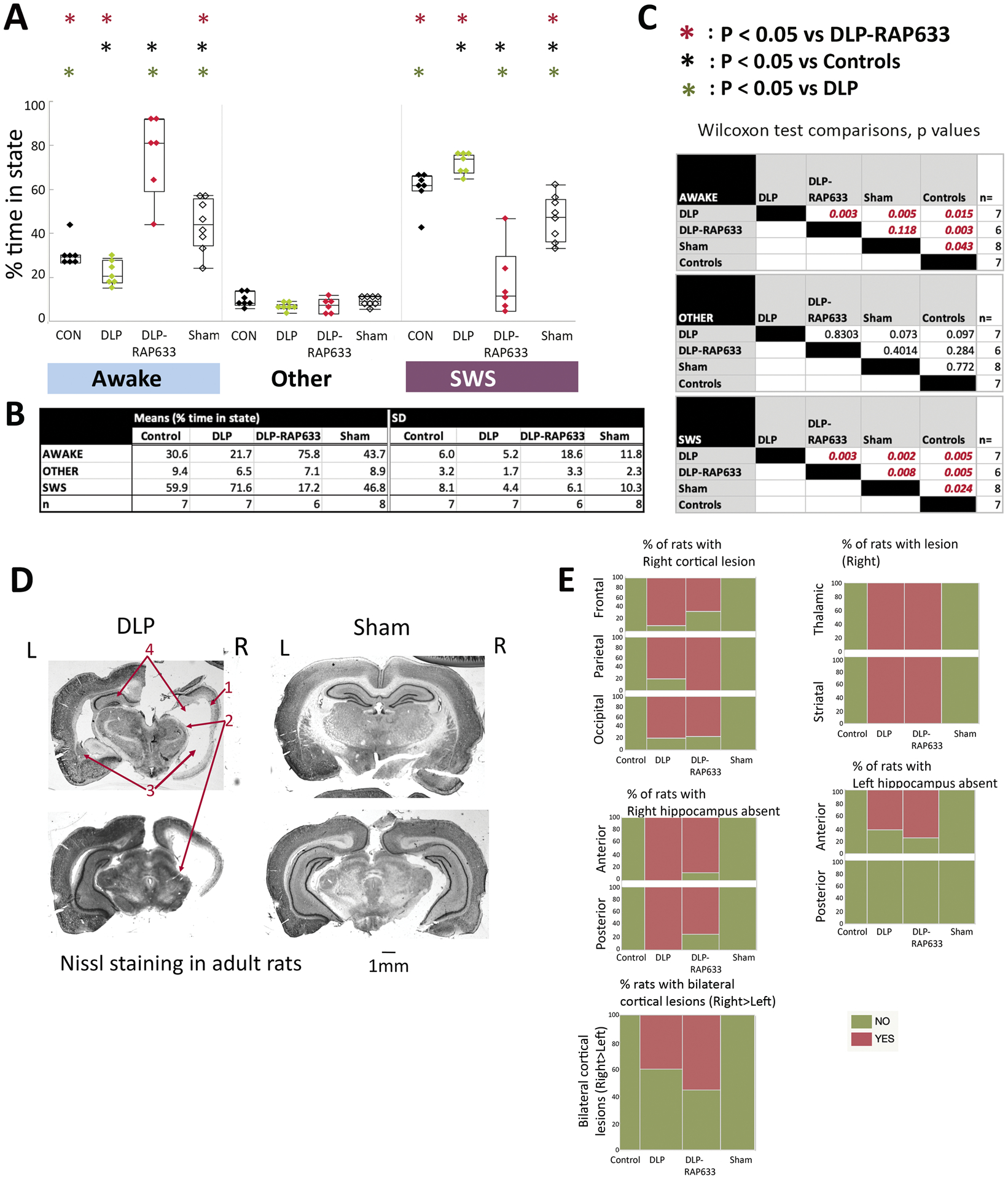
Panels A-C: Percentage of slow wave sleep (SWS) and awake state periods in DLP, DLP-RAP633, control, and sham rats during the light periods, aged PN135–177. REM and passive wakefulness were scored as “other” due to the lack of electromyographic recordings. Panels A and B present the means and standard deviations (SD) of % time spent in each state for each group. Panel C shows the nonparametric Wilcoxon test comparisons across treatment groups for each state (distributions were not normal). All studied treatment groups (DLP, DLP-RAP633, sham) were different than controls in the SWS and awake states. DLP rats had increased SWS and reduced awake states from every other group. In contrast, DLP-RAP633 had significantly increased time spent in wakefulness compared to every other group. Sham rats spent more time in awake and less in SWS state compared to either controls or DLP groups but had shorter awake periods compared to DLP-RAP633. DLP (n=7 rats); DLP-RAP633 (n=6 rats); control (n=7 rats); sham (n=8 rats).
Panel D: Nissl staining of coronal sections of adult rat brains in the DLP and sham groups. The DLP brain shows lesion at the right cortical, thalamic and striatal regions, absence of right and atrophy of left hippocampus. L: Left; R: Right. The barscale corresponds to 1mm distance. The red arrows indicate the right cerebral cortex (1), right thalamic regions (2), left striatum and corresponding region at the right (3), left hippocampus and corresponding region at the right (4).
Panel E: The distribution of injury / atrophy in the brains per each group is shown here for cortical, hippocampal, and thalamic / striatal regions or bilateral cortical lesions. Injury is classified as present (“yes”) or absent (“no”). The injury was consistently seen at the right cortical, hippocampal and striatal / thalamic periventricular regions in the DLP and DLP-RAP633 rats, unlike control and sham rats that had no injury. Detailed statistical comparisons are given in the Supplementary Table 2.
Survival
Reduced survival was observed in both DLP (53.4%) and DLP-RAP633 (56.62%) till PN60 compared to the other groups (p=0.005, Log-Rank test). Pulse rapamycin did not improve survival (Supplementary Fig 1).
Histology
Histology was performed in 9 DLP rats and 8 DLP-RAP633 rats, 8 sham, and 5 controls, using Nissl staining and uncut brains were also inspected at autopsy for visible lesions. Detailed histological analyses are shown in Fig 4 and Supplementary Table 2.
No significant injury was seen in controls or sham rats. Mild electrode imprints on the cortical region (frontal and/or central) were seen in 6/30 rats, 3 of which had SWD bursts and 4 did not. None of these had motor-type seizures.
All DLP and DLP-RAP633 showed consistent right cortical atrophy, absent right but preserved left ventral hippocampus and periventricular atrophy of right thalamus and striatum. Left dorsal hippocampus was preserved but atrophied in 3/8 DLP and 2/8 DLP-RAP633. Bilateral cortical lesions (right more than left) were seen in 40% of DLP and 55.6% of DLP-RAP633 rats ranging from parietal encephalomalacia to more extensive loss of dorsal cortico-hippocampal structures, sparing left ventral hippocampus, amygdala, diencephalon, ventral basal ganglia, and brainstem. Pulse rapamycin did not alter the histological changes in DLP rats. Among rats with such extensive brain lesions, two DLP but no DLP-RAP633 had motor-type seizures (stage 3–5).
Lesions at cortico-hippocampal and thalamic/striatal structures were more likely to be found in rats with slow-SWD and motor-type seizures. Such lesions did not affect the time in SWS (supplementary material).
DISCUSSION
We report that the multiple-hit model of IS due to structural lesions has a high rate of epilepsy in adulthood with motor-type seizures and LGS features in some of the DLP rats and that pulse rapamycin treatment for IS has antiepileptogenic effects despite the presence of cortico-hippocampal/basal ganglia structural lesions. The antiepileptogenic effect of rapamycin occurs in parallel with its effect on improving the excessive time spent in SWS seen in DLP rats, suggesting a possible mTOR co-regulation of post-IS epileptogenic and SWS-control mechanisms.
Seizure onsets and EDs lateralized to the contralateral to the injury brain, which likely reflects better volume conduction over the more preserved brain, as also reported in patients with IS1 or LGS29. Our data cannot exclude that presence of spasms contributes to the epileptogenic processes, or that stopping spasms early may stop epileptogenesis, given that pulse rapamycin also stopped IS19. The early amelioration of epilepsy severity in DLP-RAP633 by pulse rapamycin could potentially contribute to the lower rate of motor-type seizures in adulthood. This is consistent with clinical experience suggesting that early cessation of spasms may reduce rate of epilepsy and/or developmental deficits in infants with IS30–32. We have not seen any significant injury modification by rapamycin, based on our histology studies. Whether early modification of spasms severity is solely responsible for the antiepileptogenic effects of pulse rapamycin or additional mTOR-related mechanisms are implicated merits further investigation. Further, comparing the effects of early vs late treatment with pulse rapamycin would be helpful to identify the optimal therapeutic window.
DLP rats have electroclinical seizures as early as PN9–2017. Seizure monitoring has not been done between PN21–60, due to the ongoing growth of the skull at this period, and therefore it is not clear whether seizures continue or re-emerge in adulthood. Similar to DLP model, in clinical studies, almost two thirds (48–94%) of infants with IS remained with active epilepsy at longer term follow-up3, 5, 33, 34. Non-IS epileptic seizures have been reported in chronic animal models of epileptic spasms of genetic etiology, including aristaless X-linked homeobox protein (ARX)16, 35 and adenomatous polyposis cKO mouse models18, as well as in the tetrodotoxin model of epileptic spasms due to structural lesion36. Overall, induction methods that result in a chronic epilepsy phenotype with spasms also induce other types of epileptic seizures, supporting an overlap in these epileptogenic processes. In our study, sporadic focal EDs seem to be biomarkers of the underlying structural lesion or brain insult(s), rather than of active epilepsy.
The 3-day pulse rapamycin that stopped spasms, reversed the mTOR dysregulation, and improved learning in DLP rats19 also has antiepileptogenic effects. Inhibitors of mTOR showed promising results in infants with IS with tuberous sclerosis37, whereas mTOR pathway dysregulation has been implicated in other genetic or structural IS etiologies, e.g. cortical dysplasias38. The potential of mTOR inhibitors to modify the course of epileptogenesis has been demonstrated in various genetic or acquired epilepsy models, although continuous exposure to mTOR inhibitors was required38. Our findings suggest that in the setting of IS due to acquired structural lesions, mTOR inhibitors may have a stage-specific antiepileptogenic effect, targeting mTOR-driven epileptogenic processes during the acute period of spasms, that are driven either by IS or the underlying acute pathology. Given that rapamycin may suppress SWD in a genetic rat model of absence epilepsy39, the lack of rapamycin effect on slow-SWD of DLP rats may represent a pharmacoresistant trait.
The pulse rapamycin effects on spasms, post-IS epilepsy and sleep dysregulation in the DLP model suggests an interplay of post-IS epileptogenic and sleep-wake regulating networks and processes, which could be targeted by antiepileptogenic and disease modifying treatments, like rapamycin. In humans, IS often emerge during sleep-wake transitions, tonic seizures in LGS occur in sleep and interictal epileptiform activities in LGS increase in SWS2, 13, 40. Similar to our findings, rapamycin also reversed the sleep abnormalities in a mouse model of tuberous sclerosis41. In contrast, early life cannulation (sham) reduced time spent in SWS in adulthood, compared to controls, suggesting a plasticity in sleep-wake control networks during development. Interestingly, pulse rapamycin reversed the sleep/wake period ratios during light cycle in DLP rats. Sleep-wake analyses in our study was a secondary endpoint, prompted by the high association of motor-type seizures with SWS and as a result did not include electromyographic or eye movement recordings and were limited during the light cycle. Further sleep-wake studies, including such specialized recordings, as well as behavioral studies in adulthood would be needed to more accurately score the sleep-wake cycle stages and determine whether the increased wake/sleep ratio in DLP-RAP633 rats reflects an improved functional state or is pathologic.
SWD bursts have been well described in animal models of absence epilepsy (e.g. WAG/Rij and GAERS)28, 42, 43. The bilateral 7–8Hz SWDs in many naïve or sham rats is consistent with reports of SWDs in experimental controls and outbred rat strains27, 44. There are differing opinions as to whether these SWD bursts represent seizures or normal patterns, such as equivalent of human μ rhythm45. In our study, SWD bursts were typically associated with behavioral arrest or freezing when rat was awake, occurred in both wakefulness and SWS, contrary to μ rhythms, and they responded to ethosuximide (unpublished data). We therefore cannot exclude that these reflect hypersynchronous state of a network that may also contribute to absence seizures.
In clinical studies, evolution from IS to LGS occurs in 10–30%5, 33, 34. While genetic etiologies are increasingly recognized in LGS, acquired structural lesions play a role in a significant portion of LGS patients46. There are no criteria for relevant animal models of LGS. Our first long-term video-EEG studies on these models, suggest some promising similarities in the phenotype of DLP and DLP-RAP633 rats17, 19, 21 and human LGS13, including: (a) motor-type seizures from SWS, (b) slow-SWD bursts, (c) early presence of epileptic spasms, and (d) neurodevelopmental deficits which may persist in adulthood (unpublished data). In addition, some DLP rats had NCSE or more epileptic background in SWS than in AW, as also described in LGS.
The frequency of slow-SWDs in adult DLP rats is consistent with previously reported experimental models of atypical absence epilepsy [AY-9944 model and the methylazoxymethanol acetate (MAM)-AY-9944 model] with 5–6 Hz slow-SWD47. Developmental abnormalities including hippocampal heterotopias, cortical atrophy and abnormalities of cortical lamination have been reported to lead to intractable atypical absence epilepsy in rat models48. Focal cortical stroke49 and photothrombotic cortical infarcts50 demonstrate slow-SWDs (~5Hz) by enhancing excitability of thalamocortical neurons49. In our study, the slow-SWD of adult DLP rats lateralized to the hemisphere with more preserved cortico-hippocampal and thalamic structures. In human patients, atypical SWDs can be seen in epileptic encephalopathies with intractable seizures such as LGS13.
The HA α bursts at onset of motor-type seizures seen in two DLP rats resembles the generalized paroxysmal frontal α/β fast activity (GPFA) described at the onset of tonic seizures in LGS patients13, 46. In our study, HA α bursts occurred only in motor-type seizures emerging from SWS, were almost always preceded by IA. Two DLP rats also demonstrated focal beta spindles at ictal onset. In an EEG-functional MRI study, Warren et al proposed that GPFA is driven through a prefrontal cortex activation of brainstem and then thalamic structures in LGS patients of various etiologies. In our initial optimization experiments of the DLP model, rare DLP rats with spasms demonstrated bilateral cortical and hippocampal lesions (unpublished data), resembling the hydranencephaly pattern described in infants with IS51. Unexpectedly, some of the DLP rats with IS in this study (DLP and DLP-RAP633) also had dramatic cortical and hippocampal lesions, despite surviving through adulthood, two of which also manifested stage 3–6 seizures. These suggest the possible contribution of basal ganglia/brainstem structures, possibly driven by the residual ventral cortico-hippocampal structures, in the generation of IS and subsequent motor-type seizures in DLP rats.
In summary, our study supports DLP as a model of post-IS epileptogenesis and demonstrates that a pulse rapamycin treatment for IS can have antiepileptogenic effects despite the presence of structural lesions. The effects of pulse rapamycin support a central role for mTOR pathway and possibly an involvement of sleep-wake regulatory systems in post-IS epileptogenesis. Our study strongly supports the importance and potential of early interventions targeting the underlying epileptogenic processes of IS syndromes, even if due to structural lesions, when designing antiepileptogenic therapies, so as to improve prognosis.
Supplementary Material
Acknowledgments
SW Briggs acknowledges training grant awards from the Einstein CTSA award NCRR UL1-RR025750 and the NIH MSTP training grant T32-GM007288.
W Mowrey has received research grant support from NINDS U54 NS100064, US Department of Defense (W81XWH-13-1-0180) and CURE Infantile Spasms Initiative.
SL Moshé is the Charles Frost Chair in Neurosurgery and Neurology and acknowledges grant support by NIH U54 NS100064 and NS43209, U.S. Department of Defense (W81XWH-18-1-0612, W81XWH-13-1-0180), CURE Infantile Spasms Initiative, the Heffer Family and the Segal Family Foundations, and the Abbe Goldstein/Joshua Lurie and Laurie Marsh/Dan Levitz families.
AS Galanopoulou acknowledges grant support by NINDS RO1 NS091170, U54 NS100064, the US Department of Defense (W81XWH-18-1-0612, W81XWH-13-1-0180), NICHD U54HD090260, CURE Infantile Spasms Initiative, the American Epilepsy society (seed grant), and research funding from the Heffer Family and the Segal Family Foundations and the Abbe Goldstein/Joshua Lurie and Laurie Marsh/Dan Levitz families.
The authors would like to thank Hong Wang, Qianyun Li, Wei Liu and Oleksii Shandra for their excellent technical assistance.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Footnotes
Conflicts of Interest
AS Galanopoulou is Editor in Chief of Epilepsia Open and has received royalties for publications from Elsevier and Morgan and Claypool publishers.
SL Moshé is serving as Associate Editor of Neurobiology of Disease and is on the editorial board of Brain and Development, Pediatric Neurology and Physiological Research. He receives from Elsevier an annual compensation for his work as Associate Editor in Neurobiology of Disease and royalties from 2 books he co-edited. He has received consultant’s fees from UCB and Pfizer. SL Moshé holds a US patent for the multiple hit rat model (#7863499); no financial profits or conflicts have ensued as a result of this patent.
The other co-authors have no conflicts of interests to declare.
REFERENCES
- 1.Alvarez LA, Shinnar S, Moshe SL. Infantile spasms due to unilateral cerebral infarcts. Pediatrics. 1987;79:1024–6. [PubMed] [Google Scholar]
- 2.Hrachovy RA, Frost JD Jr., Kellaway P. Sleep characteristics in infantile spasms. Neurology. 1981;31:688–93. [DOI] [PubMed] [Google Scholar]
- 3.Lagae L, Verhelst H, Ceulemans B et al. Treatment and long term outcome in West syndrome: the clinical reality. A multicentre follow up study. Seizure. 2010;19:159–64. [DOI] [PubMed] [Google Scholar]
- 4.Pellock JM, Hrachovy R, Shinnar S et al. Infantile spasms: a U.S. consensus report. Epilepsia. 2010;51:2175–89. [DOI] [PubMed] [Google Scholar]
- 5.Riikonen R Infantile Spasms: Outcome in Clinical Studies. Pediatr Neurol. 2020;108:54–64. [DOI] [PubMed] [Google Scholar]
- 6.Sanmaneechai O, Sogawa Y, Silver W et al. Treatment outcomes of West syndrome in infants with Down syndrome. Pediatr Neurol. 2013;48:42–7. [DOI] [PubMed] [Google Scholar]
- 7.Wirrell EC, Shellhaas RA, Joshi C et al. How should children with West syndrome be efficiently and accurately investigated? Results from the National Infantile Spasms Consortium. Epilepsia. 2015;56:617–25. [DOI] [PubMed] [Google Scholar]
- 8.Epi4K Consortium, Epilepsy Phenome/Genome Project, Allen AS et al. De novo mutations in epileptic encephalopathies. Nature. 2013;501:217–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howell KB, Freeman JL, Mackay MT et al. The severe epilepsy syndromes of infancy: A population-based study. Epilepsia. 2021. [DOI] [PubMed] [Google Scholar]
- 10.Surana P, Symonds JD, Srivastava P et al. Infantile spasms: Etiology, lead time and treatment response in a resource limited setting. Epilepsy Behav Rep. 2020;14:100397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chu-Shore CJ, Major P, Camposano S et al. The natural history of epilepsy in tuberous sclerosis complex. Epilepsia. 2010;51:1236–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osborne JP, Lux AL, Edwards SW et al. The underlying etiology of infantile spasms (West syndrome): information from the United Kingdom Infantile Spasms Study (UKISS) on contemporary causes and their classification. Epilepsia. 2010;51:2168–74. [DOI] [PubMed] [Google Scholar]
- 13.Arzimanoglou A, Resnick T. All children who experience epileptic falls do not necessarily have Lennox-Gastaut syndrome… but many do. Epileptic Disord. 2011;13 Suppl 1:S3–13. [DOI] [PubMed] [Google Scholar]
- 14.Lux AL, Edwards SW, Hancock E et al. The United Kingdom Infantile Spasms Study (UKISS) comparing hormone treatment with vigabatrin on developmental and epilepsy outcomes to age 14 months: a multicentre randomised trial. Lancet Neurol. 2005;4:712–7. [DOI] [PubMed] [Google Scholar]
- 15.Lee CL, Frost JD Jr., Swann JW et al. A new animal model of infantile spasms with unprovoked persistent seizures. Epilepsia. 2008;49:298–307. [DOI] [PubMed] [Google Scholar]
- 16.Price MG, Yoo JW, Burgess DL et al. A triplet repeat expansion genetic mouse model of infantile spasms syndrome, Arx(GCG)10+7, with interneuronopathy, spasms in infancy, persistent seizures, and adult cognitive and behavioral impairment. J Neurosci. 2009;29:8752–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scantlebury MH, Galanopoulou AS, Chudomelova L et al. A model of symptomatic infantile spasms syndrome. Neurobiol Dis. 2010;37:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pirone A, Alexander J, Lau LA et al. APC conditional knock-out mouse is a model of infantile spasms with elevated neuronal beta-catenin levels, neonatal spasms, and chronic seizures. Neurobiol Dis. 2017;98:149–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raffo E, Coppola A, Ono T et al. A pulse rapamycin therapy for infantile spasms and associated cognitive decline. Neurobiol Dis. 2011;43:322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ono T, Moshe SL, Galanopoulou AS. Carisbamate acutely suppresses spasms in a rat model of symptomatic infantile spasms. Epilepsia. 2011;52:1678–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Briggs SW, Mowrey W, Hall CB et al. CPP-115, a vigabatrin analogue, decreases spasms in the multiple-hit rat model of infantile spasms. Epilepsia. 2014;55:94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galanopoulou AS, Mowrey WB, Liu W et al. Preclinical Screening for Treatments for Infantile Spasms in the Multiple Hit Rat Model of Infantile Spasms: An Update. Neurochem Res. 2017;42:1949–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jequier Gygax M, Klein BD, White HS et al. Efficacy and tolerability of the galanin analog NAX 5055 in the multiple-hit rat model of symptomatic infantile spasms. Epilepsy Res. 2014;108:98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katsarou AM, Li Q, Liu W et al. Acquired parvalbumin-selective interneuronopathy in the multiple-hit model of infantile spasms: A putative basis for the partial responsiveness to vigabatrin analogs? Epilepsia Open. 2018;3:155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinel JP, Rovner LI. Experimental epileptogenesis: kindling-induced epilepsy in rats. Exp Neurol. 1978;58:190–202. [DOI] [PubMed] [Google Scholar]
- 26.Akman O, Raol YH, Auvin S et al. Methodologic recommendations and possible interpretations of video-EEG recordings in immature rodents used as experimental controls: A TASK1-WG2 report of the ILAE/AES Joint Translational Task Force. Epilepsia Open. 2018;3:437–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kadam SD, D’Ambrosio R, Duveau V et al. Methodological standards and interpretation of video-electroencephalography in adult control rodents. A TASK1-WG1 report of the AES/ILAE Translational Task Force of the ILAE. Epilepsia. 2017;58 Suppl 4:10–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akman O, Demiralp T, Ates N et al. Electroencephalographic differences between WAG/Rij and GAERS rat models of absence epilepsy. Epilepsy Res. 2010;89:185–93. [DOI] [PubMed] [Google Scholar]
- 29.Yang MH, Liu J, Zhou YL et al. Asymmetric Slow-Spike-Wave Patterns with Maximal Discharges Contralateral to MRI Lesions Predict Better Surgical Prognosis in Symptomatic Lennox-Gastaut Syndrome or Lennox-Gastaut Phenotypes. Pediatr Neurosurg. 2020;55:26–35. [DOI] [PubMed] [Google Scholar]
- 30.Lombroso CT. A prospective study of infantile spasms: clinical and therapeutic correlations. Epilepsia. 1983;24:135–58. [DOI] [PubMed] [Google Scholar]
- 31.Auvin S, Hartman AL, Desnous B et al. Diagnosis delay in West syndrome: misdiagnosis and consequences. Eur J Pediatr. 2012;171:1695–701. [DOI] [PubMed] [Google Scholar]
- 32.Cohen-Sadan S, Kramer U, Ben-Zeev B et al. Multicenter long-term follow-up of children with idiopathic West syndrome: ACTH versus vigabatrin. Eur J Neurol. 2009;16:482–7. [DOI] [PubMed] [Google Scholar]
- 33.Djuric M, Kravljanac R, Tadic B et al. Long-term outcome in children with infantile spasms treated with vigabatrin: a cohort of 180 patients. Epilepsia. 2014;55:1918–25. [DOI] [PubMed] [Google Scholar]
- 34.Vendrame M, Guilhoto LM, Loddenkemper T et al. Outcomes of epileptic spasms in patients aged less than 3 years: single-center United States experience. Pediatr Neurol. 2012;46:276–80. [DOI] [PubMed] [Google Scholar]
- 35.Marsh E, Fulp C, Gomez E et al. Targeted loss of Arx results in a developmental epilepsy mouse model and recapitulates the human phenotype in heterozygous females. Brain. 2009;132:1563–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galvan CD, Hrachovy RA, Smith KL et al. Blockade of neuronal activity during hippocampal development produces a chronic focal epilepsy in the rat. J Neurosci. 2000;20:2904–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samueli S, Dressler A, Groppel G et al. Everolimus in infants with tuberous sclerosis complex-related West syndrome: First results from a single-center prospective observational study. Epilepsia. 2018;59:e142–e6. [DOI] [PubMed] [Google Scholar]
- 38.Galanopoulou AS, Gorter JA, Cepeda C. Finding a better drug for epilepsy: the mTOR pathway as an antiepileptogenic target. Epilepsia. 2012;53:1119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Russo E, Citraro R, Donato G et al. mTOR inhibition modulates epileptogenesis, seizures and depressive behavior in a genetic rat model of absence epilepsy. Neuropharmacology. 2013;69:25–36. [DOI] [PubMed] [Google Scholar]
- 40.Galanopoulou AS, Bojko A, Lado F et al. The spectrum of neuropsychiatric abnormalities associated with electrical status epilepticus in sleep. Brain Dev. 2000;22:279–95. [DOI] [PubMed] [Google Scholar]
- 41.Zhang B, Guo D, Han L et al. Hypothalamic orexin and mechanistic target of rapamycin activation mediate sleep dysfunction in a mouse model of tuberous sclerosis complex. Neurobiol Dis. 2020;134:104615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Onat FY, van Luijtelaar G, Nehlig A et al. The involvement of limbic structures in typical and atypical absence epilepsy. Epilepsy Res. 2013;103:111–23. [DOI] [PubMed] [Google Scholar]
- 43.Powell KL, Tang H, Ng C et al. Seizure expression, behavior, and brain morphology differences in colonies of Genetic Absence Epilepsy Rats from Strasbourg. Epilepsia. 2014;55:1959–68. [DOI] [PubMed] [Google Scholar]
- 44.Taylor JA, Reuter JD, Kubiak RA et al. Spontaneous Recurrent Absence Seizure-like Events in Wild-Caught Rats. J Neurosci. 2019;39:4829–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kelly KM. Spike-wave discharges: absence or not, a common finding in common laboratory rats. Epilepsy Curr. 2004;4:176–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Warren AEL, Harvey AS, Vogrin SJ et al. The epileptic network of Lennox-Gastaut syndrome: Cortically driven and reproducible across age. Neurology. 2019;93:e215–e26. [DOI] [PubMed] [Google Scholar]
- 47.Cortez MA, Perez Velazquez JL, Snead OC 3rd. Animal models of epilepsy and progressive effects of seizures. Adv Neurol. 2006;97:293–304. [PubMed] [Google Scholar]
- 48.Serbanescu I, Cortez MA, McKerlie C et al. Refractory atypical absence seizures in rat: a two hit model. Epilepsy Res. 2004;62:53–63. [DOI] [PubMed] [Google Scholar]
- 49.Paz JT, Davidson TJ, Frechette ES et al. Closed-loop optogenetic control of thalamus as a tool for interrupting seizures after cortical injury. Nat Neurosci. 2013;16:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kelly KM, Kharlamov A, Hentosz TM et al. Photothrombotic brain infarction results in seizure activity in aging Fischer 344 and Sprague Dawley rats. Epilepsy Res. 2001;47:189–203. [DOI] [PubMed] [Google Scholar]
- 51.Neville BG. The origin of infantile spasms: evidence from a case of hydranencephaly. Dev Med Child Neurol. 1972;14:644–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


