Abstract
Objective
To investigate the role of autologous platelet‐rich plasma (PRP) on the repair of meniscal white‐white zone injury through promoting the proliferation of canine bone marrow‐derived mesenchymal stem cells (BMSCs).
Methods
A total of 24 beagle dogs were selected to construct meniscal white‐white zone injury models in both lateral knee joints. All subjects were divided into four groups: control, BMSCs, PRP, and PRP + BMSCs. Immunohistochemistry was applied in the expression detection of type I and type II collagens. HE staining and methylene blue staining were performed to observe the injury of cartilage of lateral femoral condyle in each group. ELISA was used to detect the osteopontin (OPN) content in cartilage of lateral femoral condyle. HE staining and magnetic resonance imaging (MRI) were used to observe the healing of meniscus in each group. Outcome measures include the expression of OPN in the synovial fluid of knee joint, the expression of type I collagen and type II collagen, the healing of meniscus injury, and the damage degree of lateral femoral condyle cartilage.
Results
Compared with the control group, the expressions of type I and type II collagens were enhanced in the PRP group and the PRP + BMSCs group. Compared with 1 week before modeling, the expression of OPN was elevated in the control group and the BMSCs group at 3 weeks after modeling. There were no significant differences in the above indicators between the PRP group and the PRP + BMSCs group. According to MRI and pathological section after HE staining, meniscal healing in the PRP group and the PRP + BMSCs group was significantly improved as compared to that of the control group and the BMSCs group (all P < 0.05), and there was no significant difference between the PRP group and the PRP + BMSCs group (P > 0.05). All subjects were divided into the non‐healing group and the healing group in accordance with the HE staining results in previous experiment. The injury of cartilage of lateral femoral condyle was significantly heavier in the non‐healing group than that in the healing group.
Conclusion
The application of PRP alone or in combination with BMSCs could promote the clinical healing rate of meniscal white‐white zone injury.
Keywords: Mesenchymal Stem Cells, Osteoarthritis, Osteopontin, Platelet‐Rich Plasma, Tibial Meniscus Injury
The study investigates the role of autologous platelet‐rich plasma (PRP) on the repair of meniscal white‐white zone injury through promoting the proliferation of canine bone marrow‐derived mesenchymal stem cells (BMSCs).
The application of PRP alone or in combination with BMSCs could promote the clinical healing rate of meniscal white‐white zone injury.
Introduction
Menisci were once wrongly identified as “functionless remnants in intra‐articular leg muscles,” and the importance of meniscus was not noted until Fairbank first proposed the role of the menisci in load transmission across the knee in 1947 1 . Meniscal tears are the most commonly‐seen type of knee injuries and are a leading cause of knee disability, accounting for no less than 15% of all knee injuries 2 . According to the location of meniscal tears, they are divided into three main types: red‐red tears, red‐white tears, and white‐white tears, among which white‐white tears are located in the inner third region without blood supply, and the meniscus tears that extend into the avascular region (white‐white tears) are the most difficult to manage 3 . The management of meniscal injuries has continued to evolve, and the elevated degenerative changes of the knee due to total meniscectomy were discovered, resulting in increased commitment among orthopaedists to avoid total meniscectomy whenever possible 4 . Therefore, this paper explores new treatment other than meniscectomy in the healing of meniscal white‐white zone.
Bone marrow‐derived mesenchymal stem cells (BMSCs), an important part of chondrogenesis, are known as an attractive cell source of cartilage regenerative medicine due to the fact that they are easy to isolate and expand in a minimally invasive manner, thus BMSCs‐based cell therapy is increasingly accepted as a potential treatment of cartilage defect 5 . Moreover, platelet‐rich plasma (PRP) has raised a great deal of interest in recent years, as an autologous concentration of platelets in small plasma volume, which is considered to be capable of promoting angiogenesis and increasing the healing rate of ligament, skeletal muscle, and bone 6 . Platelet‐rich plasma (PRP) provides a release of various growth factors which are involved in tissue repair processes, including vascular endothelial growth factor (VEGF), platelet derived growth factor (PDGF), and transforming growth factor β (TGF‐β) 7 . Furthermore, it is well‐documented in previous clinical and animal studies that a combination of PRP and autogenous bone graft could improve the rate of osteogenesis and accelerate bone formation qualitatively, indicating that different concentrations of PRP might influence bone formation in a different manner 8 , 9 . However, there are limited data about the effects of different concentrations of PRP on the growth and proliferation of BMSC at present; therefore, this study aims to investigate the effects of PRP on the healing of meniscal white‐white zone through inducing the canine BMSCs.
Materials and Methods
Ethical Statement
Animal experiments were designed with the consent of the animal ethics committee and all the studies were conducted strictly in accordance with international standards.
Preparation of Dog Platelet‐Rich Plasma (PRP)
A total of 24 healthy adult male beagle dogs (provided by the laboratory animal department at Xiangya Hospital of Central South University) were chosen. The dogs weighed 15–17 kg each and were raised in the clean‐level breeding center. After anesthesia using Sumianxin II combined with pentobarbital, about 40 ml of whole venous blood was drawn from the forearm vein, and 2 ml were taken from this sample for whole blood platelet count, and the remaining blood was used to make PRP by a secondary centrifugation method (Fig. 1A) 10 . Firstly, the sample was centrifuged at 200 g for 10 min, and the whole blood was divided into three layers. After discarding the supernatant higher than 3 mm above the interface, the remaining supernatant down to 3 mm below the interface was collected and transferred to another centrifuge tube, which was centrifuged at 200 g for 10 min. At this time, the liquid was divided into two layers, and the supernatant higher than 3 mm above the interface was discarded. After shaking, the remaining material was PRP. All operating processes were kept sterile. The numbers of platelets in the whole blood and PRP blood were counted by an automatic blood analyzer (Perlong Medical, Nanjing, China). After adding 10% solution of calcium chloride and 100 U/ml of thrombin, PRP was mixed by shaking and placed for 6–10 s to make a PRP gel, which was stored in sterile conditions at 4°C for future use.
Fig. 1.
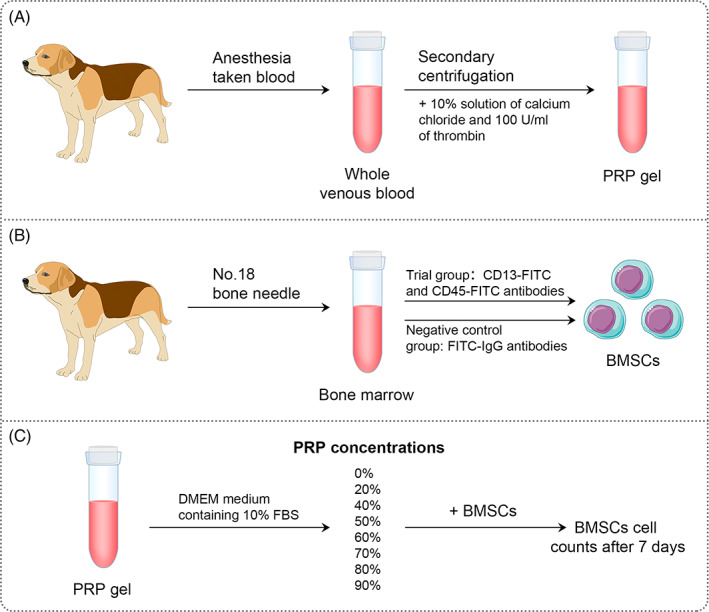
Preparation of dog platelet‐rich plasma (A) and culture and identification of BMSCs (B) and BMSCs cultured in different concentrations of PRP (C).
Culture and Identification of BMSCs
After the dogs were anesthetized, a No.18 bone needle was used to withdraw 2 ml of bone marrow from the femur. The bone marrow was then placed in a test tube with heparin and 6 ml of DMEM culture medium containing 10% FBS was added. After mixing, the sample was placed into a 25 ml flask and cultured in an incubator at 37°C and 5% CO2. The medium was changed every 2 days. When the degree of confluency of BMSCs reached about 90%, they were digested with 0.25% trypsin and centrifuged for subculture. The fourth generation of BMSCs were digested by trypsin and their concentration was adjusted to 1 × 106/ml, and 1 ml of the cells were placed in an EP tube, followed by dyeing with the addition of CD13‐FITC and CD45‐FITC antibodies (all from Becton, Dichkinson and Company, Franklin Lakes, NJ, USA), respectively. In the negative control, FITC‐IgG antibody was added, followed by mix and incubation in the dark for 30 min. And then, the cells were resuspended in PBS after centrifugation (Fig. 1B). Using a flow cytometer (Becton, Dichkinson and Company, Franklin Lakes, NJ, USA), 1000 cells were detected and the percentage of positive cells was analyzed with Cellquest software.
BMSCs Cultured in Different Concentrations of PRP
PRP stored at 4°C was taken out and dissolved at room temperature. After counting the total number of platelets through the automatic blood analyzer, DMEM medium containing 10% FBS was used to make different PRP concentrations (20%, 40%, 50%, 60%, 70%, 80%, and 90%). BMSCs were inoculated in 35‐mm culture plates at 2 × 105 cells/plate, and medium with different concentrations of PRP was added. A DMEM medium with 10% FBS‐containing but no PRP (0% PRP) was used as control. On day 7, cell proliferation ability was measured through cell counts (Fig. 1C).
Meniscal White‐White Zone Injury Model Establishment and Grouping
In all of the 24 beagle dogs, the lateral meniscal white‐white zone injury models were established in both knees. Specific steps were as follows: The beagle dogs were anesthetized and put into a lateral position, and a medial patellar incision was made next to the middle line of the knee. The patella was then dislocated towards the outside direction, and a part of the fat pad was removed. By peeling off the lateral collateral ligament near the end point of femoral, the lateral meniscus was exposed (Fig. 2A). In the inner white‐white zone of the lateral meniscus, a small sharp knife was used to make full‐layer damage with about 2 cm in length, and the meniscus incision was closed with absorbable sutures (3–0). All beagle dogs were divided into four groups with each group containing six beagle dogs. In the saline control group (control), 2 ml of saline was used to treat the suture; in the BMSCs group, 2 ml of BMSCs suspension was used to treat the suture; in the PRP group, 2 ml of PRP gel was placed in the gap of the suture; in the PRP + BMSCs group, 1 ml of PRP and 1 ml of BMSCs (PRP and BMSCs were diluted and mixed to a suitable concentration for BMSCs growth) were used to treat the suture (Fig. 2B). For contralateral knee operation, the steps were the same as above. After the operation, intramuscular injection of gentamicin was done for 3 days, and the knee joints in bilateral lower extremity were immobilized with gyp for 2 weeks. The wound dressings at the incision sites were replaced in general ways, and the stitches were removed after 7–10 days.
Fig. 2.
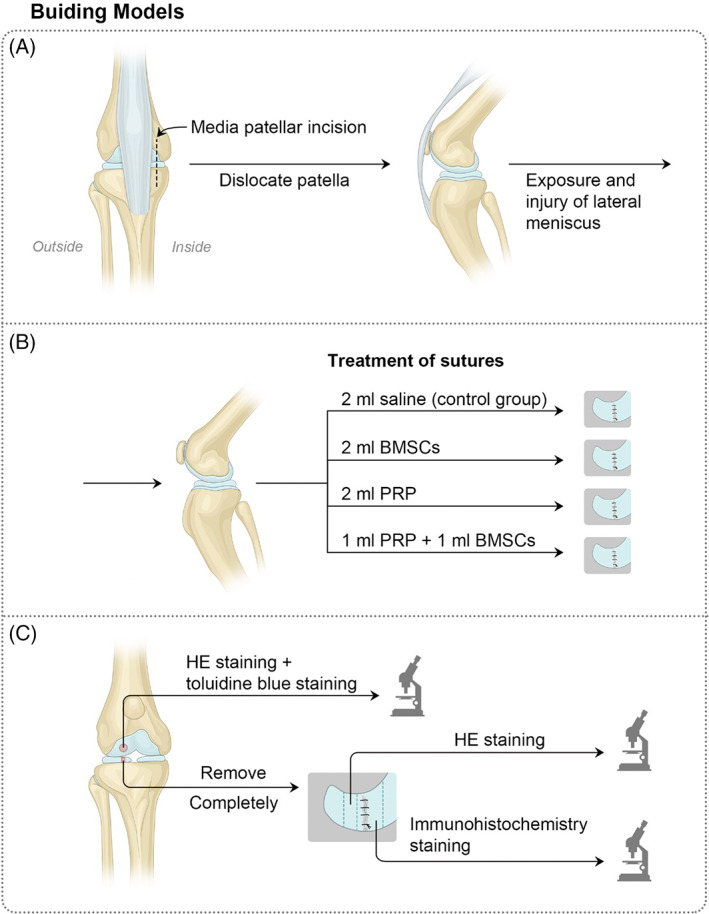
Meniscal white‐white zone injury model establishment and grouping (A, B) and tissue selection and observation (C).
Imaging Examination
At 3 months after the operation, knee joint MRI (GE, Fairfield city, CT, USA) was done for checking the conditions of meniscal healing in each group. Beagle dogs were examined referring to the examination settings of human tissues after anesthesia.
Tissue Selection
At 3 months after the operation, beagle dogs were killed by anesthesia overdose, and a cut was made through the original surgical incision. General observation about meniscal healing was made at first, and the meniscus was removed completely. After fixing in 4% paraformaldehyde, dehydrated, and embedded in paraffin, serial sections with a thickness of 3 μm were produced, which were baked at 62°C for 60 min and stored for future use. The lateral sections at the central portion of meniscus were used for hematoxylin–eosin (HE) staining to observe the meniscal healing, and the sections near the damaged area were chosen for immunohistochemistry to measure the synthesis of collagen. Sagittal sections near the weight‐bearing area at central portion of the lateral femur were used for HE staining and toluidine blue staining to observe the cartilage damage at femoral condyles (Fig. 2C).
HE Staining
After dewaxing and hydration, the sections were stained with hematoxylin for 5 min and washed with tap water for 1 min. Subsequently they were differentiated in 1% hydrochloric acid alcohol for 30 s and soaked in tap water for 15 min, then stained with 0.5% eosin for 3 min and slightly rinsed in distilled water. Finally, the sections were dehydrated, made transparent, mounted in neutral resin, and observed under an optical microscope for pathological morphology of the meniscus.
Immunohistochemistry Staining
The sections were dewaxed and hydrated, washed with PBS, and incubated in 3% H2O2 for 10 min at room temperature. They were then washed with PBS and distilled water, respectively, and pepsin solution was added dropwise and incubated for 10 min at 37°C. After washing with PBS, normal goat serum was used to block the antigen, and the sections were incubated at room temperature for 15 min. After removal of the serum completely, anti‐dog type I collagen and type II collagen monoclonal antibody (both with 1: 500 dilution, purchased from Sigma, Saint Louis, MO, USA) were added dropwise, respectively. After incubation at 4°C overnight, the sections were washed with PBS for 3 times, and biotinylated goat anti‐rabbit IgG secondary antibody was added dropwise and incubated at 37°C for 15 min. After PBS washing, working solution of streptavidin labeled with horseradish peroxidase was added dropwise and incubated at 37°C for 20 min. After washing with PBS, the sections were developed in DAB chromogenic reagent for 10 s, washed with tap water, and re‐stained with hematoxylin for 2 min. Finally, after washing, the sections were differentiated with hydrochloric acid alcohol for a few seconds, washed with tap water, dehydrated, made transparent, and mounted in a neutral resin.
Toluidine Blue Staining
After the sections were dewaxed and hydrated conventionally, they were stained with 2% toluidine blue (Sigma, Saint Louis, MO, USA) for 5 min and rinsed with tap water for 2 min. They were then dehydrated with gradient alcohol, made transparent by xylene, and mounted in a neutral resin.
The Expression of OPN in the Synovial Fluid of Knee Joint
OPN is an important bone matrix protein, which is closely related to the formation and development of bone. Studies have shown that the expression of OPN in subchondral bone of osteoarthritis (OA) patients is positively correlated with the degree of disease.
A total of 0.5 ml intra‐articular synovial fluid was extracted from each beagle dog at 1 week before modeling and 3 months after modeling, and the OPN concentrations were measured using a canine OPN ELISA assay kit (Sigma, Saint Louis, MO, USA). After serial dilution of the standard, 100μl of sample to be tested were added into a microplate well, followed by set of a control well. After incubation at 37°C for 2 h, the liquid was discarded, and 100 μl of testing solution A was added for incubation at 37°C for 1 h. After discarding the liquid, the plate was washed three times, and 100 μl of detection reagent B were added, followed by laminating and incubation at 37°C for 1 h. After that, the plate was washed five times with the addition of 50 μl of stop solution (the blue color will immediately change to yellow color) and the optical density (OD) value of each well was detected at a wavelength of 450 nm using a microplate reader.
The Expression of Type I Collagen and Type II Collagen
Collagen has the effect of promoting chondrogenesis, so the determination of its expression is helpful to evaluate the degree of cartilage formation. Type I collagen is found in bones and skin. It accounts for more than 90% of bone organic matter, and is also the main component of skin, tendons, and ligaments. Type II collagen is the main cartilage collagen.
After immunohistochemistry staining, the collagen expression in meniscus was observed under a stereo microscope and photographed, wherein a brown staining was associated with a positive staining result. The quantitative analysis of collagen expression was done using Image Pro Plus 6.0 image analysis software (Media Cybernetics, Rockville, Maryland, USA), and the surface density was calculated as the ratio between the target area and the statistical field area. The numerical density was calculated as the ratio between the target number and the statistical field area, and the perimeter density was calculated as the ratio between the target perimeter and statistical field area. Three sections from each sample were analyzed and averaged.
The Healing of Meniscus Injury
MRI is often used to evaluate the injury or healing of meniscus. Meniscal healing was evaluated according to the Stoller method 11 : grade I is associated with abnormal signals that are spherical or ovoid and are not connected to articular surface; degree II is associated with abnormal signals that are linear but are not connected to articular surface; degree III is associated with high linear signals connected to articular surface, or meniscus signals appear at abnormal positions, or the meniscus signal is missing. Among them, grade I and II are associated with meniscal healing while grade III is not associated with meniscus healing.
Histopathological sections can also be used to evaluate the healing of meniscus. After HE staining, the pathological morphology of meniscus was observed under light microscope. Evaluation of meniscal healing was done referring to the method reported by Henning 12 : non‐healing is defined as less than 50% healing; partial healing is defined as 50%–90% healing; healing is defined as greater than 90% healing.
The Damage Degree of Lateral Femoral Condyle Cartilage
Lateral meniscus injury often leads to lateral femoral condylar cartilage injury. After HE staining and toluidine bule staining, the degree of cartilage injury can be determined by observing the pathological section of the injured area under light microscope.
The damage to cartilage of lateral femoral condyle in each group was observed under an optical microscope. Histopathological grading was done according to the Mankin scoring of cartilage 13 : normal cartilage: 0–2 points; mild cartilage damage: 2–6 points; moderate cartilage damage: 7–10 points; severe cartilage damage: 11–14 points.
Statistical Analysis
Data analysis was done using SPSS 21.0 statistical software (SPSS, Inc., Chicago, IL, USA). Measurement data were presented as mean ± standard deviations ( ± s). Comparisons between two groups were done using the Bonferroni test, and the comparisons between before and after the operation within a group were done using the paired t‐test; categorical data were presented using composition ratios, and the comparisons between two groups were done using the chi‐square test. A P value of <0.05 was considered statistically significant.
Results
Effect of PRP on the Growth Ability of BMSCs
The analysis results from the automatic blood analyzer showed that the platelet concentration was (158.89 ± 35.23) × 109/L in the whole blood of beagle dogs and was significantly lower than the platelet concentration [(1368.67 ± 52.51) × 109/L] in prepared PRP (P < 0.05). The primary bone marrow cells from beagle dogs were extracted and cultured, and cell colonies could be seen after 5–7 days, and in about 14 days the cells completely covered the bottom of tissue culture flasks. The spindle‐formed fibroblast‐like cells grow rapidly. The fourth generation of BMSCs were inspected with flow cytometry, and the results showed that the expression of CD13 was positive, while the expression of CD45 was negative (Fig. 3A), indicating that high purity BMSCs were obtained with a natural purification method. The cell counting results (Fig. 3B) showed that when the PRP concentration was less than 70%, the proliferation of BMSCs gradually increased with an increasing PRP concentration, and the proliferation ability of BMSCs was the highest at a PRP concentration of 70%. When the concentration of PRP was greater than 70%, the effect of increasing PRP concentration on promoting BMSCs proliferation gradually decreased, and at a PRP concentration of 90%, the proliferation ability of BMSCs was not statistically different from that of the control group (0% PRP) (P > 0.05), indicating that the PRP concentration had great influence on BMSCs proliferation. Therefore, the PRP concentration of 70% was chosen as the appropriate concentration for further in vivo intervention experiments.
Fig. 3.
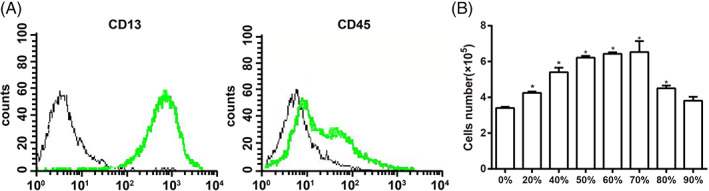
Expressions of BMSCs surface antigens were inspected using flow cytometry (A) and histogram that shows the effect of different PRP concentrations on the growth ability of BMSCs (B). Note: *, indicates P < 0.05 as compared with the 0% PRP group.
Comparisons of the Healing of Meniscal White‐White Zone Injury by MRI Among Four Groups
After establishing the meniscal white‐white zone injury model, the beagle dogs in each group showed no wound infection, and the wound healed well; all beagle dogs in each group were alive healthily with no significant changes in body weight, body length, and height before and after the modeling. Meniscal healing results from MRI showed that: at 3 months after the operation, the intra‐articular effusion in the control group was basically eliminated, and the high signal of meniscus reached until the articular surface; in the BMSCs group, there was a slight intra‐articular effusion, and the high signal in the lateral meniscus was in contact with the articular surface; in the PRP group and the PRP + BMSCs group, there was no intra‐articular effusion, the lateral meniscus was intact, and there was no abnormal signal (Fig. 4A). The results of meniscal white‐white zone healing evaluated by the Stoller method showed: there was no healing in the control group; the number of cases of healing in the BMSCs group was one; the number of cases of healing in the PRP group was nine; and the number of cases of healing in the PRP + BMSCs group was 10. The chi‐square test analysis showed that: meniscal healing in the PRP group and the PRP + BMSCs group was significantly improved as compared to that of the control group and the BMSCs group (all P < 0.05), and there was no significant difference between the PRP group and the PRP + BMSCs group (P > 0.05) (Fig. 4B), indicating that PRP can promote the healing of meniscal white‐white zone injury in beagle dogs, and the effect of PRP alone was not different from that of using PRP and BMSCs jointly.
Fig. 4.
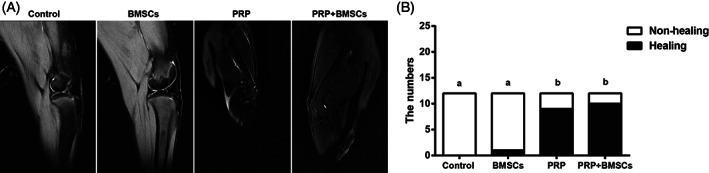
Comparisons of MRI images of beagle dogs among four groups (A) and histogram for the evaluation of meniscal white‐white zone healing by MRI (B). Note: Different lowercase letters indicate P < 0.05 as compared between the two groups.
Comparisons of Histopathology of Meniscus Among Four Groups
By visual observation and using a blunt object to tentatively penetrate or slightly pull the healing site in the meniscal white‐white zone in a lateral direction showed that, in the control group and the BMSCs group, there was essentially no continuous tissues through the meniscus injury; in both the PRP group and the PRP + BMSCs group, there were continuous tissues through meniscus at the lesion site and the tissues have been repaired, and the injury site could not be clearly determined by the naked eye (Fig. 5A). The HE staining of the meniscal tissue sections showed that there was no healing in the control group and the BMSCs group and there were two separations at the outer edge of the damaged site; in the PRP group and the PRP + MSCs group, the healing of meniscus was obvious and continuous fibers were seen through the lesions, and there was no histological differences between the callus and nearby meniscus tissues (Fig. 5B). Assessment of meniscal white‐white zone healing using HE staining showed: there was no complete healing in the control group; there was one complete case of healing in the BMSCs group, nine complete cases of healing in the PRP group, and 10 complete cases of healing in the PRP + BMSCs group. The chi‐square test analysis showed that: as compared with the meniscal white‐white zone healing in the control group, there was no significant difference in the BMSCs group (P > 0.05), but the situations in the PRP group and the PRP + BMSCs group were improved significantly (all P < 0.05), and there was no significant difference in the healing between the PRP and BMSCs + PRP groups (P > 0.05) (Fig. 5C). This further confirmed that using PRP alone or in combination with BMSCs can promote the repair of meniscal white‐white zone in beagle dogs.
Fig. 5.
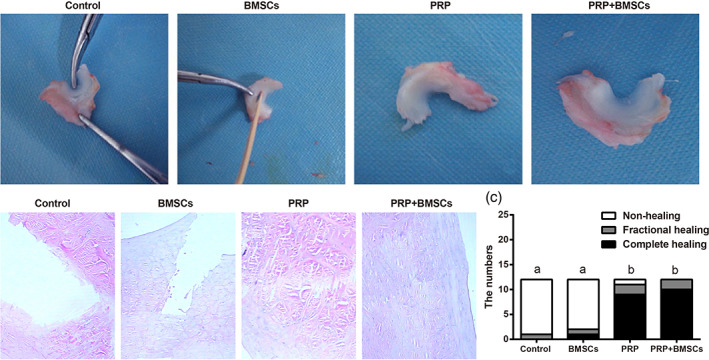
General observation of meniscus in the beagle dogs of each group (A), HE staining observation of beagle dogs among four groups (B) and histogram evaluation of meniscal white‐white zone healing by HE staining (C) Note: Different lowercase letters indicate P < 0.05 as compared between the two groups.
Comparisons of Type I Collagen and Type II Collagen Expressions of Meniscus Among Four Groups
Immunohistochemical staining of type I collagen and type II collagen of meniscus has shown that, the expressions of type I collagen and type II collagen in the control group and the BMSCs group was relatively weak, while the expressions of type I collagen and type II collagen in the PRP group and the PRP + BMSCs group were stronger with more brown staining (Fig. 6A,C). Quantitative analysis on the numerical density, surface density, and perimeter density of type I collagen and type II collagen expressions in each group showed that: as compared with the different parameters in the control group, there were no significant differences in the BMSCs group (all P > 0.05), but the parameters in the PRP group and the PRP + BMSCs group all significantly increased (all P < 0.05). In addition, there were no significant differences among those three parameters in the expressions of type I collagen and type II collagen between the PRP group and BMSCs + PRP group (all P > 0.05) (Fig. 6B,D). This indicates that PRP intervention promotes collagen expression during the repair of meniscal white‐white zone in beagle dogs.
Fig. 6.
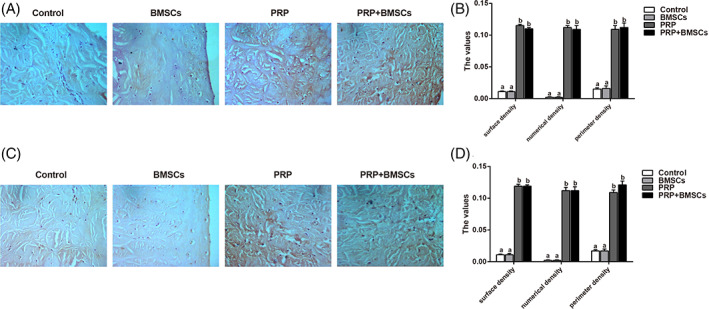
Immunohistochemical staining results of type I and type II collagens in the meniscus of different groups (A, C) and histogram of protein quantitative analysis (B, D) Note: Different lowercase letters indicate P < 0.05 as compared between the two groups.
Comparisons of OPN Contents in the Synovial Fluid of Knee Joint Among Four Groups
Results of ELISA showed that at 1 week before modeling, the OPN content in the synovial fluid of knee joint in each group was not significantly different (all P > 0.05). At 3 months after modeling, there was no significant difference between the control group and the BMSCs group (P > 0.05), but the OPN content in the PRP group and the PRP + BMSCs group significantly decreased (P < 0.05). In addition, there was no significant difference between the PRP group and the PRP + BMSCs group (P > 0.05). Compared with the OPN content at 1 week before the modeling, the OPN content in the control group and the BMSCs group was significantly increased at 3 months after the modeling (all P < 0.05), but the contents in the PRP and the PRP + BMSCs groups did not change significantly (P > 0.05) (Table 1). This suggests that PRP inhibits the increase of OPN concentration in the synovial fluid of the knee joint caused by meniscus injury.
TABLE 1.
Comparisons of OPN contents in the synovial fluid of knee joint among four groups (Mean ± SD, pg./ml)
| Control group | BMSCs group | PRP group | BMSCs + PRP group | |
|---|---|---|---|---|
| One week before modeling | 1998.61 ± 35.15 | 2003.23 ± 25.76 | 2026.00 ± 53.78 | 2012.90 ± 47.78 |
| Three months after modeling | 5024.95 ± 82.27 # | 4002.18 ± 60.74 # | 2221.77 ± 113.16 * | 2140.63 ± 138.86 * |
indicates P < 0.05 as compared with the control group.
indicates P < 0.05 as compared with that at 1 week before modeling.
Impact of Meniscal White‐White Zone Injury on Cartilage of Lateral Femoral Condyle
According to the results of HE staining shown above, the experimental animals were divided into: the non‐healing group (22 knees) and the healing group (a total of 26 knees including the fully healed and partially healed knees). HE and toluidine blue staining of pathological tissues of lateral femoral condyle showed that, in the non‐healing group, the surface at the injury site of cartilage of lateral femoral condyle was arranged in disorder with chondrocyte hypertrophy: the staining was uneven; the tide line was not continuous, or a part of the tide line was lost; cracking was seen in a portion of cartilage surface; the toluidine blue staining was uneven and some parts were not stained. In the healing group, the cartilage cells were arranged in neat rows, and there was no obvious hypertrophy or vacuolization: the stain was uniform, and the tidal line was continuous and neat; and the toluidine blue staining was uniform (Fig. 7A). The histopathological grading results on cartilage damage showed that, in the non‐healing group, the cartilages in six knees were normal, while the cartilages in 16 knees had mild injuries. In the healing group, the cartilages in 20 knees were normal, while the cartilages in six knees had mild injuries. There were no moderate or severe cartilage damage in either group. Statistical analysis showed a significant difference in the cartilage damage between the two groups (P < 0.001) (Fig. 7B). This indicates that the unhealed meniscus white‐white zone injury will lead to femoral cartilage injury in the corresponding knee joint.
Fig. 7.
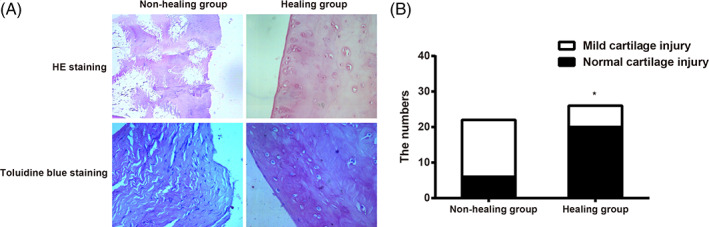
HE and toluidine blue staining of cartilage of lateral femoral condyle in the healing and non‐healing groups (A) and histopathological grading histogram of numbers of cartilage damage between the healing and non‐healing groups (B). Note: *, indicates P < 0.05 as compared with the non‐healing group.
Discussion
The crucial roles of the menisci in human knee are well‐documented, occupying 60% of the contact area between the surfaces of tibial and femoral cartilage, and more than 50% of joint compression forces are transmitted by menisci 14 . Avascular meniscal tears, also known as meniscal white‐white tears, have a poor self‐healing ability. As poor long‐term clinical results following partial and total meniscectomy have been recorded by many investigators, PRP has gained much attention as a promising nonoperative treatment for the repair of meniscal tears extended into the avascular zone 15 , 16 . Therefore, the present study investigates the efficacy of PRP on the healing of meniscal white‐white zone by inducing BMSCs.
First of all, we find that PRP could remarkably promote the proliferation of canine BMSCs, and both PRP and PRP + BMSCs treatments are effective in the repair of meniscal white‐white zone. BMSCs are cells postulated to be involved in the process of tissue homoeostasis, remodeling, and repair through ensuring the mature cell replacements are free of physiological turnover, senescence, injury, or disease 17 . In recent years, the potential of BMSCs in achieving good healing through the formation of fibrocartilage‐like tissue and improvement in biomechanical properties has raised much concern, thus MSCs‐based therapies for the regeneration of different tissues have gained popularity 18 . PRP is also a widely tested medicine because of its potential in aiding tissue regeneration with low self‐healing, including white‐white meniscal tears 19 . Furthermore, former study also demonstrates that the PRP concentration variations might affect the bone formation, possibly due to the fact that when a small amount of BMSCs are mixed with a large volume of PRP, part of the bone cells are transferred in the autograft, which contains the primary bone regenerating cells; at the same time, the bone cells would be exposed to high PRP concentrations 8 . In other words, the proliferation of MSCs are suppressed by high PRP concentrations, but were upregulated by low PRP concentrations. This is why PRP in appropriate concentration combined with BMSCs is an effective regimen for the healing of meniscal white‐white zone 20 . We innovatively proposed that PRP in the concentration of 70% promotes the proliferation of canine BMSCs the most based on our intensive experiments, in the hope of setting a recommended concentration of PRP as a guideline for later investigators.
In addition, our results indicate that the expressions of type I collagen and type II collagen during meniscus injury repair process are significantly promoted and the concentration of OPN in synovial fluid induced by meniscus injury is significantly inhibited in the PRP group and the PRP + BMSCs group. PRP is known for its role in hemostasis where they can prevent blood loss at the injury sites, and PRP contains a great number of growth factors, including PDGF, TGF‐β, VEGF, as well as cytokines, such as PF4 and CD40L proteins 21 . A recent study about tissue biology has recorded the regulatory roles of growth factors in tissue repair and indicates the effectiveness for application of growth factors in tissue healing. For instance, the study shows that TGF‐β can significantly increase the expression of collagens, which is consistent with our results 22 . OPN is a kind of phosphoprotein secreted by many cell types, including bone‐forming cells and hypertrophic chondrocytes. It is also pointed out that elevated OPN level in synovial fluid and articular cartilage is linked with increased disease severity of knee osteoarthritis patients 23 . Intra‐articular injection of PRP can stimulate chondrogenesis and generation of glycosaminoglycan, thus OPN expression level is inhibited during the process of chondrogenesis 24 . Moreover, the findings of our study revealed that the incidence of cartilage injury is significantly higher in the non‐healing group. Cartilage injury is often accompanied with meniscus tears, and the increased articular cartilage damage rate has been identified as a cause of the delay between injury and surgical reconstruction 25 .
All in all, PRP in the concentration of 70% has the most promoting ability in the proliferation of canine BMSCs. Besides, PRP can significantly upregulate the expressions of collagens and downregulate the concentration of OPN in synovial fluid. Therefore, PRP injection may be a promising approach in the repair of meniscal white‐white zone. However, the most suitable composition of PRP is still unknown, thus further study should be conducted for understanding the PRP to obtain the maximum value of promoting the repair of damage.
Consent for publication
Written informed consent for publication was obtained from all participants.
Author Contributions
Y.‐s.L. and L.‐c.W. designed the experiment. W.‐f.X. conducted the experiment and wrote the article. Yun‐tao Yang collected the data. W.‐f.X. and M.H. did the statistical analysis. D.L., Z.‐j.C., and D.‐j.Y. provided critical review of the article.
Disclosure: The authors declare that they have no conflicts of interest pertaining to this study.
Grant Sources: This work was supported by Provincial Natural Science Foundation of Hunan (No.2020JJ3060, 2018JJ2636), Provincial Clinical Medical Technology Innovation Project of Hunan (No.2020SK53709), the Administration of Traditional Chinese Medicine of Hunan Province (No.2021075), Innovation‐Driven Project of Central South university (No.2020CX045), Wu Jieping Medical Foundation (320.6750.2020‐03‐14), CMA Young and Middle‐aged Doctors Outstanding Development Program‐‐Osteoporosis Specialized Scientific Research Fund Project (G‐X‐2019‐1107‐12), the Clinical and Rehabilitation Research Foundation of Xiangya Hospital and Weiming of Peking University (xywm2015II04) and the Key program of Health Commission of Hunan Province (20201902).
Contributor Information
Yu‐sheng Li, Email: liyusheng@csu.edu.cn.
Li‐cheng Wei, Email: weilicheng78@163.com.
References
- 1. Lee SJ, Aadalen KJ, Malaviya P, et al. Tibiofemoral contact mechanics after serial medial meniscectomies in the human cadaveric knee. Am J Sports Med, 2006, 34: 1334–1344. [DOI] [PubMed] [Google Scholar]
- 2. Baek J, Sovani S, Glembotski NE, et al. Repair of avascular meniscus tears with electrospun collagen scaffolds seeded with human cells. Tissue Eng Part A, 2016, 22: 436–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Noyes FR, Barber‐Westin SD. Management of meniscus tears that extend into the avascular region. Clin Sports Med, 2012, 31: 65–90. [DOI] [PubMed] [Google Scholar]
- 4. Lubowitz JH, Verdonk PC, Reid JB 3rd, et al. Meniscus allograft transplantation: a current concepts review. Knee Surg Sports Traumatol Arthrosc, 2007, 15: 476–492. [DOI] [PubMed] [Google Scholar]
- 5. Koga H, Shimaya M, Muneta T, et al. Local adherent technique for transplanting mesenchymal stem cells as a potential treatment of cartilage defect. Arthritis Res Ther, 2008, 10: R84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mifune Y, Matsumoto T, Takayama K, et al. The effect of platelet‐rich plasma on the regenerative therapy of muscle derived stem cells for articular cartilage repair. Osteoarthr Cartil, 2013, 21: 175–185. [DOI] [PubMed] [Google Scholar]
- 7. de Vos RJ, Weir A, van Schie HT, et al. Platelet‐rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. Jama, 2010, 303: 144–149. [DOI] [PubMed] [Google Scholar]
- 8. Choi BH, Zhu SJ, Kim BY, Huh JY, Lee SH, Jung JH. Effect of platelet‐rich plasma (PRP) concentration on the viability and proliferation of alveolar bone cells: an in vitro study. Int J Oral Maxillofac Surg, 2005, 34: 420–424. [DOI] [PubMed] [Google Scholar]
- 9. Fennis JP, Stoelinga PJ, Jansen JA. Mandibular reconstruction: a histological and histomorphometric study on the use of autogenous scaffolds, particulate cortico‐cancellous bone grafts and platelet rich plasma in goats. Int J Oral Maxillofac Surg, 2004, 33: 48–55. [DOI] [PubMed] [Google Scholar]
- 10. Landesberg R, Roy M, Glickman RS. Quantification of growth factor levels using a simplified method of platelet‐rich plasma gel preparation. J Oral Maxillofac Surg, 2000, 58: 297–300; discussion −1. [DOI] [PubMed] [Google Scholar]
- 11. Stoller DW, Martin C, Crues JV 3rd, Kaplan L, Mink JH. Meniscal tears: pathologic correlation with MR imaging. Radiology, 1987, 163: 731–735. [DOI] [PubMed] [Google Scholar]
- 12. Henning CE, Lynch MA, Clark JR. Vascularity for healing of meniscus repairs. Art Ther, 1987, 3: 13–18. [DOI] [PubMed] [Google Scholar]
- 13. Liu R, O'Connell M, Johnson K, Pritzker K, Mackman N, Terkeltaub R. Extracellular signal‐regulated kinase 1/extracellular signal‐regulated kinase 2 mitogen‐activated protein kinase signaling and activation of activator protein 1 and nuclear factor kappaB transcription factors play central roles in interleukin‐8 expression stimulated by monosodium urate monohydrate and calcium pyrophosphate crystals in monocytic cells. Arthritis Rheum, 2000, 43: 1145–1155. [DOI] [PubMed] [Google Scholar]
- 14. Noyes FR, Barber‐Westin SD, Chen RC. Repair of complex and avascular meniscal tears and meniscal transplantation. Instr Course Lect, 2011, 60: 415–437. [PubMed] [Google Scholar]
- 15. Zellner J, Hierl K, Mueller M, et al. Stem cell‐based tissue‐engineering for treatment of meniscal tears in the avascular zone. J Biomed Mater Res B Appl Biomater, 2013, 101: 1133–1142. [DOI] [PubMed] [Google Scholar]
- 16. Shin KH, Lee H, Kang S, et al. Effect of leukocyte‐rich and platelet‐rich plasma on healing of a horizontal medial meniscus tear in a rabbit model. Biomed Res Int, 2015, 2015: 179756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Horie M, Sekiya I, Muneta T, et al. Intra‐articular injected synovial stem cells differentiate into meniscal cells directly and promote meniscal regeneration without mobilization to distant organs in rat massive meniscal defect. Stem Cells, 2009, 27: 878–887. [DOI] [PubMed] [Google Scholar]
- 18. Dutton AQ, Choong PF, Goh JC, et al. Enhancement of meniscal repair in the avascular zone using mesenchymal stem cells in a porcine model. J Bone Joint Surg Br, 2010, 92: 169–175. [DOI] [PubMed] [Google Scholar]
- 19. Kon E, Filardo G, Delcogliano M, et al. Platelet‐rich plasma: new clinical application: a pilot study for treatment of jumper's knee. Injury, 2009, 40: 598–603. [DOI] [PubMed] [Google Scholar]
- 20. Nagata MJ, Messora M, Pola N, et al. Influence of the ratio of particulate autogenous bone graft/platelet‐rich plasma on bone healing in critical‐size defects: a histologic and histometric study in rat calvaria. J Orthop Res, 2010, 28: 468–473. [DOI] [PubMed] [Google Scholar]
- 21. Anitua E, Andia I, Ardanza B, Nurden P, Nurden A. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost, 2004, 91: 4–15. [DOI] [PubMed] [Google Scholar]
- 22. Filardo G, Kon E, Della Villa S, Vincentelli F, Fornasari PM, Marcacci M. Use of platelet‐rich plasma for the treatment of refractory jumper's knee. Int Orthop, 2010, 34: 909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lipton A, Brown GA, Mundy GR, et al. New opportunities for the management of cancer‐related bone complications. Clin Adv Hematol Oncol, 2009, 7: 1–30 quiz 1. [PubMed] [Google Scholar]
- 24. Zhang W, Yuan Z, Pei X, Ma R. In vivo and in vitro characteristic of HIF‐1alpha and relative genes in ischemic femoral head necrosis. Int J Clin Exp Pathol, 2015, 8: 7210–7216. [PMC free article] [PubMed] [Google Scholar]
- 25. Krych AJ, Sousa PL, King AH, Engasser WM, Stuart MJ, Levy BA. Meniscal tears and articular cartilage damage in the dislocated knee. Knee Surg Sports Traumatol Arthrosc, 2015, 23: 3019–3025. [DOI] [PubMed] [Google Scholar]


