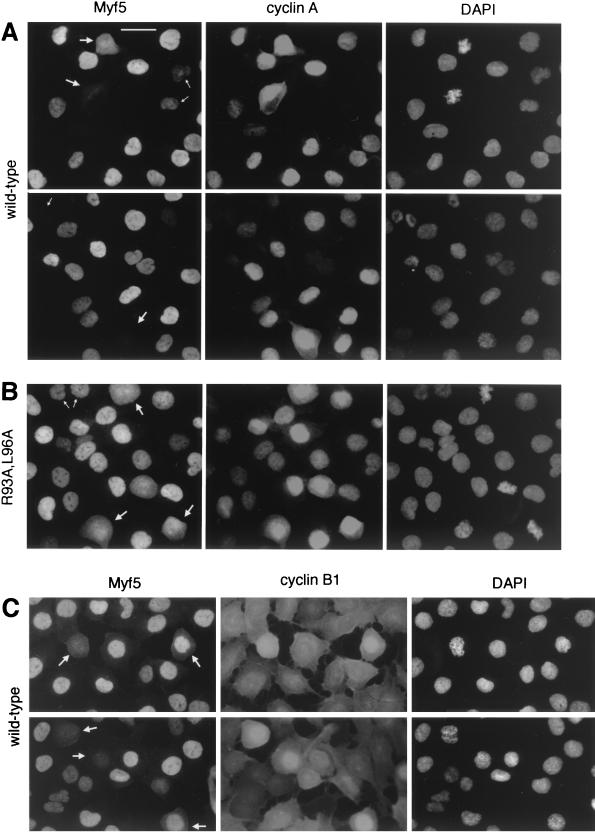
FIG. 4.
Cell-by-cell analyses of Myf5 levels at mitosis. UTA6-Myf5/ cells were prepared as described for Fig. 3A and, 12 h after release from double thymidine block, were fixed and prepared for immunofluorescence analyses of Myf5 and cyclin A (A and B) or Myf5 and cyclin B1 (C). Bar, 25 μm. (A) UTA6-Myf5/wt cells costained for Myf5, cyclin A, and DAPI. Two fields are shown. Most cells show strong nuclear staining for cyclin A, confirming that the majority of cells are in late S phase, G2, or prophase. The redistribution of cyclin A throughout the cell is observed following nuclear-envelope breakdown at the end of prophase, and its levels decrease gradually during subsequent prometaphase and metaphase (31). Cells with decondensed chromatin and which show no cyclin A staining are presumed to be in early G1 (having completed mitosis), and indeed, these cells systematically occur in pairs (top, small arrows). As expected, there is a clear correlation between the intensities of Myf5 and cyclin A stainings in cells outside of mitosis. That is, Myf5 staining is strongest in cells showing strong nuclear cyclin A staining (but before the onset of nuclear condensation). Cyclin A-negative cells in late mitosis or G1 (bottom and top, respectively; small arrows) typically show faint or no staining for Myf5. By contrast, in prophase and prometaphase cells (large arrows), there is no clear correlation between Myf5 and cyclin A stainings. (B) UTA6-Myf5/R93A,L96A cells costained for Myf5, cyclin A, and DAPI. Small arrows, G1 cells; large arrows, prometaphase cells. (C) UTA6-Myf5/wt cells costained for Myf5, cyclin B1, and DAPI. Cyclin B1 translocates to the nucleus from the cytoplasm during prophase (31). Cells clearly in late prophase or prometaphase (showing nuclear cyclin B1, or in which nuclear-envelope breakdown has occurred) are indicated with arrows.