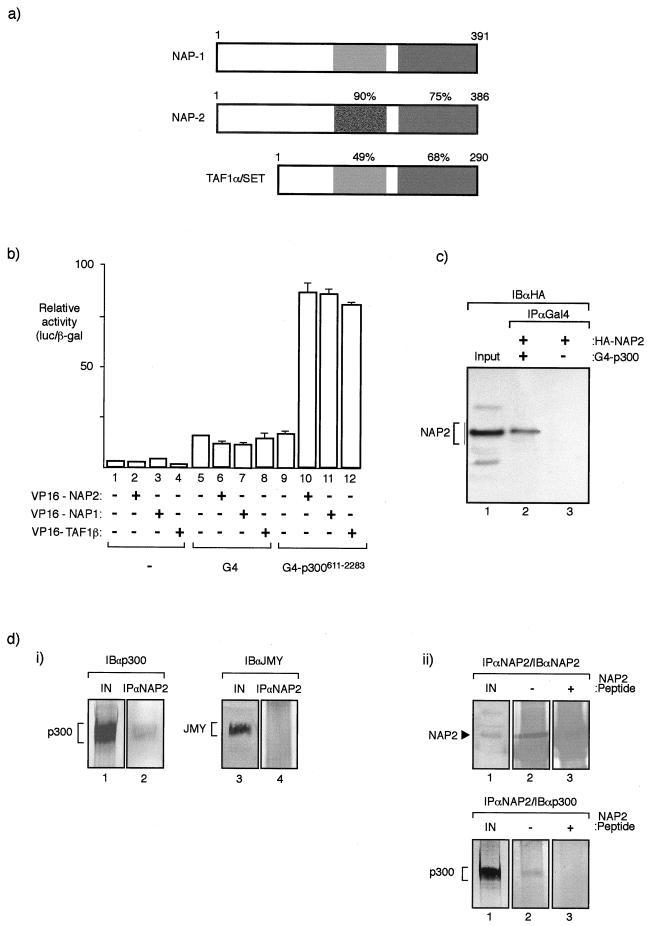
FIG. 1.
NAP proteins interact with p300. (a) Diagrammatic summary of members of the NAP family (NAP1, NAP2, and TAF1α) and the level of similarity between each human protein. The protein regions were compared with NAP2 from residue 159 to 270 and residue 337 to 382. The corresponding regions in NAP1 were 167 to 278 and 344 to 387, and in TAF they were 91 to 189 and 243 to 288. It should be noted that TAF1α and TAF1β are proteins that arise through alternative splicing and differ in a region in the N-terminal domain (42). (b) Mammalian two-hybrid assay performed in U2OS cells where the indicated VP16-NAP or TAF1β expression vectors (0.5 μg) were transfected together with expression vectors encoding G4 or pG4-p300611–2283 (0.5 μg). The values shown represent the average of three separate readings and the ratio of luciferase derived from the reporter pG4-luc and the internal control pCMV-βgal. (c) Coimmunoprecipitation of NAP2 and p300 hybrid proteins from U2OS cells transfected with pG4-p300611–2283 (lane 2) or pG4 (lane 3) together with pCMV-HA-NAP2 (lanes 1, 2, and 3). After extraction, immunoprecipitation was performed with anti-Gal4 antibody (IPαGal4) followed by immunoblotting with anti-HA (12CA5) (IBαHA). An extract from cells transfected with HA-NAP2 is shown in lane 1. (d) Panel i, coimmunoprecipitation of endogenous NAP2 and p300 from murine A31 cell extracts was performed using anti-NAP2 peptide antiserum followed by immunoblotting with either anti-p300 (lane 2) or, as a control, anti-JMY (lane 4). Lanes 1 and 3 show the input extract. Note that p300, but not JMY (47), is present in the NAP2 immunoprecipitate and further that A31 cell extracts were used because the anti-NAP2 antibody recognizes murine NAP2 only. Panel ii, coimmunoprecipitation of endogenous NAP2 and p300 from A31 cells was performed using anti-NAP2 peptide antiserum in the presence (+) (lane 3) or absence (−) (lane 2) of homologous peptide followed by immunoblotting with either anti-NAP2 (top) or anti-p300 (bottom). Lane 1 shows the input extract, and NAP2 and p300 are indicated. Note that the NAP2 polypeptide is absent in the presence of the competing NAP2 peptide (+) (top) and that p300 in the immunoprecipitate correlates with the presence of NAP2 (bottom).