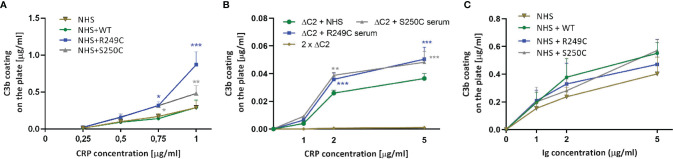
Figure 2.
C3b deposition assay in the presence of CRP and human Ig preparation. (A) Increasing concentrations of C-reactive protein (CRP) were coated onto the ELISA microplate and overlaid with 0.25% normal human serum (NHS) +/− recombinant C2 variants. After 30 min incubation, C3b deposition was detected by anti-C3b antibody. Purified C3b directly coated on the plate was used as a standard. (B) The same assay was performed with a 1:1 mix of ΔC2 serum with patients’ sera or NHS as a control. (C) ELISA plates were coated with a preparation of human Ig (pentaglobin) instead of CRP and overlaid with NHS with recombinant C2 variants. Differences between the given C2 variant and the WT were analyzed by Dunnett multiple comparison test for non-repeated measures. *P < 0.05; **P < 0.01; ***P < 0.001.