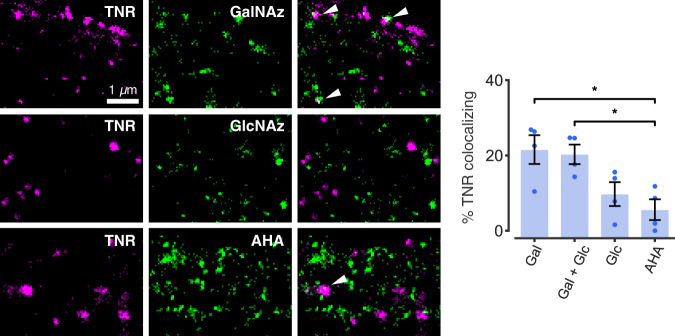
Fig. 8. TNR recycling possibly relates to TNR re-glycosylation.
Newly O-glycosylated proteins were labeled by feeding neurons with azide-modified galactosamine (GalNAz) and/or glucosamine (GlcNAz), which were then revealed by click chemistry. Alternatively, newly-synthesized proteins were labeled by feeding neurons with azidohomoalanine (AHA), which was also tagged using click chemistry. Newly-emerged TNR epitopes were labeled 6 h post-blocking and visualized at the surface. The neurons were imaged with 2-color-STED. Scale bar = 1 µm. Quantification of the colocalization of the signals confirmed that internalized TNR epitopes colocalize significantly with GalNAz or GalNAz+GlcNAz, at levels substantially higher than the minimal AHA colocalization, which is not significantly different from negative controls (relying on non-specific primary antibodies). N = 4 independent experiments, ≥10 neurons per datapoint. Kruskal-Wallis test (H3 = 9.022, *p = 0.029), followed by two-sided Fisher’s LSD (*p = 0.014 and *p = 0.021 for ‘GalNAz’ and ‘GalNAz+GlcNAz’ respectively). Data represent mean ± SEM, dots indicate individual experiments. Source data are provided in Source Data file.