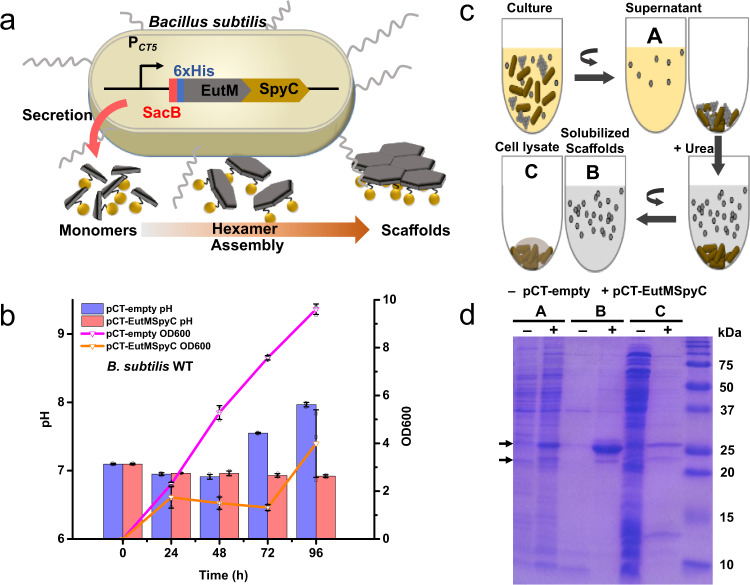
Fig. 2. Establishment of self-assembling scaffold building block secretion.
a Self-assembling EutM scaffold building blocks containing a C-terminal SpyCatcher (SpyC) domain and a SacB secretion signal sequence followed by a His-tag are expressed and secreted by B. subtilis under the control of a cumate inducible promoter. b Growth and pH of cultures expressing EutMSpyC was followed for 96 h in SMM at 20 °C and compared to empty plasmid control cultures. Data are shown as mean values ± SD and error bars represent the standard deviations of three independent biological replicate cultures. Colored bars and lines represent mean values. Black symbols represent data points and error bar. c Self-assembling scaffolds settle with cells, requiring their solubilization from cell pellets by a gentle wash with 4 M urea (see Methods for details). d Protein expression and secretion after 48 h of cultivation were analyzed by SDS-PAGE where: (A) is the culture supernatant (10-fold concentrated by TCA precipitation), (B) urea supernatant after scaffold solubilization, and (C) resulting cell pellet after lysozyme treatment for analysis of remaining protein. Arrows indicate EutM-SpyCatcher bands. Expected sizes are 24.4 kDa and 21.1 kDa with and without SacB secretion signal peptide, respectively. The shown data is representative of three independent biological replicate cultures. Source data are provided as a Source Data file.