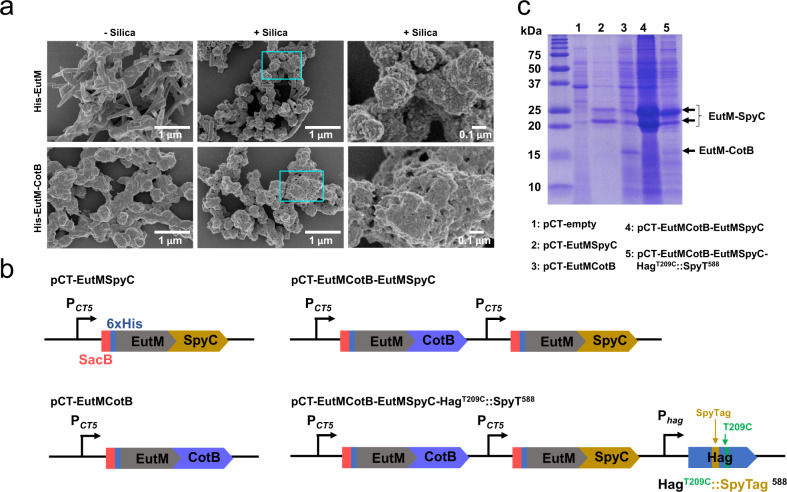
Fig. 5. Implementing silica biomineralization by scaffolds.
a Silica biomineralization by His-EutM and His-EutM-CotB scaffolds expressed and purified from E. coli was investigated by SEM following the incubation of 1 mg/mL protein without (left column) and with (center columns) 100 mM silica for 2 h at room temperature. Right columns show 10-fold magnification of marked regions. Images shown are representative of one set of purified proteins. b Different plasmids were constructed for EutM-CotB expression and secretion in B. subtilis alone or together with EutM-SpyCatcher as shown. For final ELM fabrication, a consolidated plasmid was designed for the co-expression of EutM-SpyCatcher and EutM-CotB with HagT209C::SpyTag588 in B. subtilis ΔlytC ΔflhG. Each gene is expressed by its own promoter (cumate inducible PCT5 promoter, native hag promoter). c Expression and secretion of EutM-CotB by B. subtilis ΔlytC ΔflhG was investigated and compared to EutM-SpyCatcher. Urea solubilized fractions were prepared from cultures transformed with different expression plasmids and grown as in Fig. 2. Except for pCT-EutMSpyC (lane 2), all urea fractions from the different recombinant cultures were concentrated 10-fold by TCA precipitation prior to SDS-PAGE analysis. Data shown are representative of three independent experiments. Source data are provided as a Source Data file.