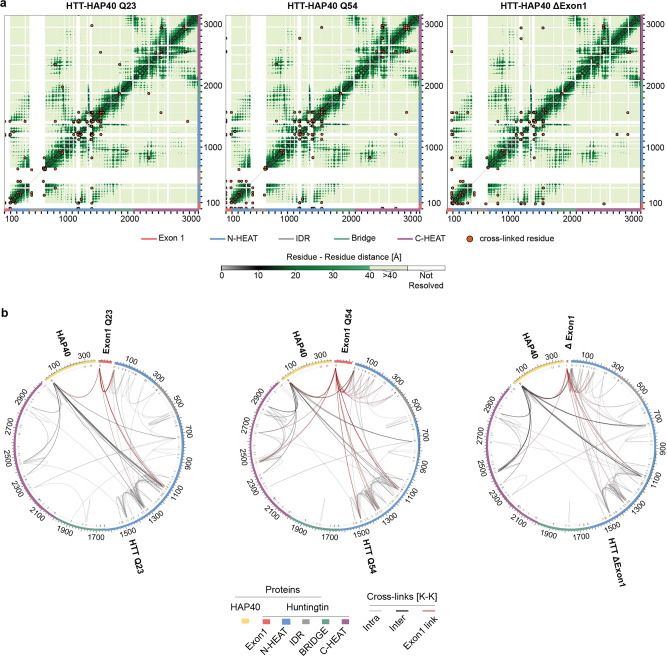
Fig. 5. Exon 1 is highly flexible and conformationally dynamic in the context of the full-length protein.
a Mapping cross-linked sites to the HTT-HAP40 sequence of different samples, with cross-linked residue pairs shown as orange circles. Intramolecular distances for HTT-HAP40 (PDBID: 6X9O) shown from grey to green as per the coloured scale bar with unmodelled regions of the protein shown in white. b Mapping cross-links to the HTT-HAP40 sequence of different samples, with exon 1 in red, N-HEAT in blue, bridge domain in green, IDR in grey, C-HEAT in purple and HAP40 in yellow. Cross-linked lysine residues are indicated in red and unmodified lysine residues are indicated in black on the numbered sequence. Intermolecular cross-links (HTT-HAP40) are shown in black, intramolecular cross-links (HAP40-HAP40 or HTT-HTT) are shown in grey and exon 1 cross-links are shown in red. All residues following the exon 1 region of the different constructs are numbered the same for clarity.