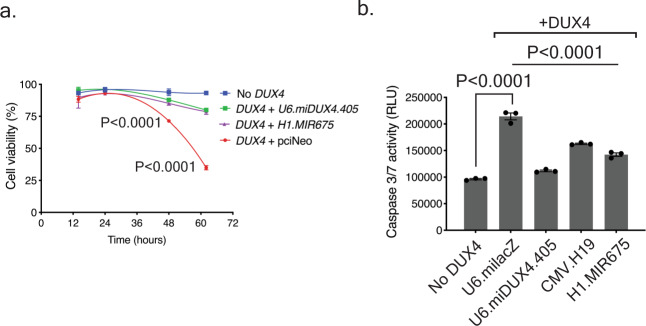
Fig. 4. miR-675 protects cells from DUX4-induced cell death.
a Cell viability assay in HEK293 cell transfected with indicated plasmids. Cell death was measured by counting Trypan blue-stained cells over the indicated time course and calculated by subtracting the number of dead cells from the total number of cells plated per well. No significant difference in cell viability was seen before 36 h of transfection. Cells co-transfected with DUX4 and the control pciNeo plasmid showed 23 ± 2% (P < 0.0001) and 58 ± 1% (P < 0.0001) cell death by 48 and 62 h, respectively, when compared to the mock-transfected cells (No DUX4). However, co-transfection with DUX4 and H1.MIR675 or U6.miDUX4.405 protected HEK293 cells from DUX4-induced cell death, as only 9 ± 2% and 6 ± 2% of cells were dead by 48 h, respectively, and slightly increased by 62 h. These values were not significantly different from mock-transfected HEK293s but represented significant protection from cell death compared to the DUX4 + pciNeo control (P < 0.0001 for both H1.MIR675 and U6.mi405). Two-way ANOVA followed by Tukey’s multiple comparison tests were performed for statistical analyses. Results were reported as the mean cell viability ratio ± SEM (N = 3 independent experiment). b Caspase-3/7 assay 48 hrs after co-transfection of HEK293 cells with DUX4 expression plasmid and either U6.miLacZ, U6.miDUX4.405, H1.MIR675, or CMV.H19. The U6.miLacZ negative control failed to protect cells from Caspase-3/7 activation (2.2 ± 0.1-fold increase; P < 0.0001) compared to mock-transfected cells. In contrast, Caspase-3/7 activity was significantly decreased in cells transfected with DUX4 and U6.miDUX4.405, H1.MIR675 and CMV.H19 (49 ± 1%, 34 ± 2% and 24 ± 2%, respectively; P < 0.0001). Two-way ANOVA followed by Dunnett’s multiple comparison tests were performed for statistical analyses. Results reported as mean relative luminescence units (RLU) ± SEM (N = 3 independent experiment). Source data are provided as a Source data file.