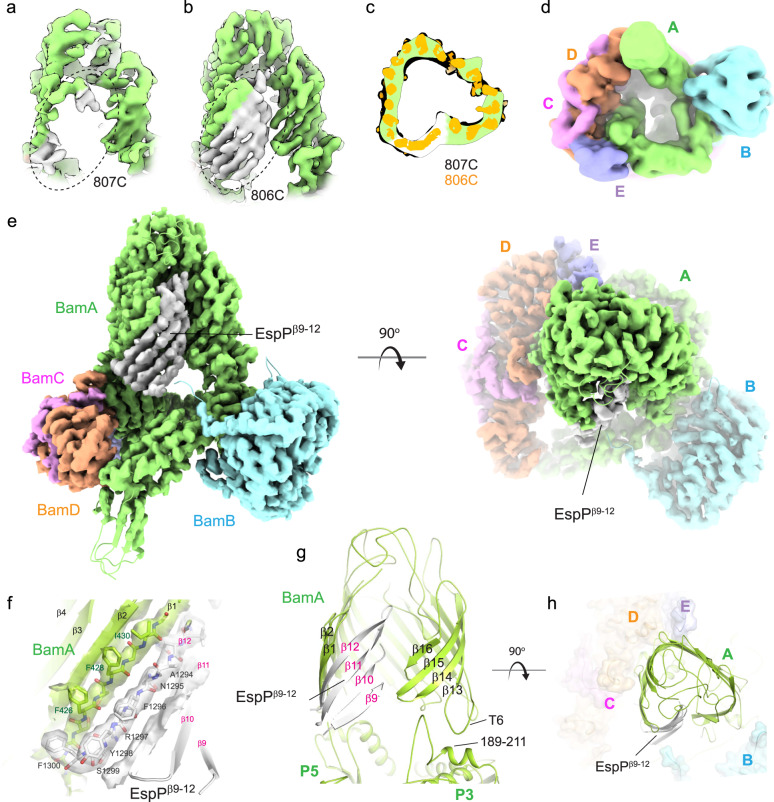
Fig. 8. High-resolution structure of the BAM/MBP-EspPβ9–12 hybrid-barrel early intermediate.
CryoEM map along the lateral seam within the BAM/MBP-EspPβ9–12 structure with crosslinking between a residue 807 of β16 of BamA (green) and residue 1226 of β9 of EspP (gray) and b residue 806 of β16 of BamA and residue 1226 of β9 of EspP. The dashed oval shows the increase in order observed with crosslinking at residue 806 of BamA. c A top-down cutaway view comparing the non-sharpened map from panel a (green is the BamA barrel; white is EspPβ9–12) with the map in panel b (orange), depicting the same state observed for both crosslinking sites. d A bottom view of a down-filtered map (7 Å) from panel b; no extra density is observed as was seen for the structure from the in vitro assay (Fig. 6f–i). e Orthogonal views of the 3.4 Å resolution MBP-EspPβ9–12 cryoEM map/structure showing the integration of EspPβ9–12 within the expanded barrel of BamA, forming a hybrid-barrel early intermediate. The last strand of EspPβ9–12 (β12) pairs with the first strand of BamA (β1). The N-terminal strands of EspPβ9–12 then curve towards the inside of the barrel, which would assist in sealing the hybrid-barrel during biogenesis to maintain membrane integrity. f Zoomed view of the four strands of EspPβ9–12 integrated into the barrel of BamA. The density for β1 of BamA and β12 of EspPβ9–12 is shown as a transparent isosurface, allowing unambiguous assignment of the residues. g View of the BamA/EspPβ9–12 barrel with strand arrangements. The interaction between turn 6 (T6) and residues 189-211 of POTRA5 (P3) is shown which may stabilize the expansion of the C-terminal region of the BamA barrel during OMP biogenesis. h Zoomed-in view of the triangular shape of BamA/EspPβ9–12 hybrid-barrel, showing excellent agreement with the low-resolution structure from Fig. 6j.