Abstract
As closed-loop insulin therapies emerge into clinical practice and evolve in medical research for type 1 diabetes (T1D) treatment, the limitations in these therapies become more evident. These gaps include unachieved target levels of glycated hemoglobin in some patients, postprandial hyperglycemia, the ongoing need for carbohydrate counting, and the lack of non-glycemic benefits (such as prevention of metabolic syndrome and complications). Multiple adjunct therapies have been examined to improve closed-loop systems, yet none have become a staple. Sodium-glucose-linked cotransporter inhibitors (SGLTi’s) have been extensively researched in T1D, with average reductions in placebo-adjusted HbA1c by 0.39%, and total daily dose by approximately 10%. Unfortunately, many trials revealed an increased risk of diabetic ketoacidosis, as high as 5 times the relative risk compared to placebo. This narrative review discusses the proven benefits and risks of SGLTi in patients with T1D with routine therapy, what has been studied thus far in closed-loop therapy in combination with SGLTi, the potential benefits of SGLTi use to closed-loop systems, and what is required going forward to improve the benefit to risk ratio in these insulin systems.
Keywords: type 1 diabetes, SGLT inhibitors, artificial pancreas, closed-loop therapy, adjunct therapy
Introduction
Type 1 diabetes (T1D), which accounts for 5%-10% of cases of diabetes, 1 is marked by the lifelong need for intensive insulin therapy at diagnosis, which often occurs before adulthood. The goal of intensive insulin therapy is to achieve glycated hemoglobin (HbA1c) levels of ≤7.0%, as reflected in multiple guidelines, to reduce complications.2-5 Optimal control is only achieved by a few with T1D worldwide, specifically 21% in the United States.6-10
Closed-loop insulin systems are the most advanced device-based treatment in T1D care,11,12 and with systems such as the MiniMed 670G®, Tandem’s Control-IQ®, CamDiab®, and DiabeLoop®, they are now part of clinical practice.13,14 Hybrid closed-loop therapy (HCL), compared to routine therapy, increase time-in-range (TIR), reduce time in hypoglycemia, improve HbA1c, and increase patient satisfaction.15-21 Unfortunately, HCL has not been able to perfect T1D care; in the iDCL trial using Control-IQ®, there remained almost half of the participants who had HbA1c levels above 7%. 20
A possible way to rectify this problem is the use of adjuvant pharmacotherapy, which has been previously assessed as adjunct to standard of care and HCL.17,19,22-28 Metformin has some glycemic improvement, but has not been tested with HCL. 29 Glucagon-like Peptitde-1 (GLP1)-receptor agonists have demonstrated conflicting outcomes in benefits as adjunct to T1D care, both with and without HCL.24-27,30-33 The effect of dipeptitdyl peptidase-4 inhibitors is not as significant compared to other adjunctive agents in T1D, 34 with one inpatient study demonstrating reduced post-prandial glycemia with sitagliptin using HCL. 27 While pramlintide has been studied as an injectable pharmacotherapy adjunct to routine insulin therapy or integrated into HCL,19,24,25 and has shown benefits,22,24 its clinical application is limited by the lack of an existing co-formulation with insulin and the need for separate injections or infusion systems.
A separate group of medications is the sodium-glucose-linked cotransporter inhibitors (SGLTi’s). Sodium-glucose-linked cotransporters (SGLTs) are solute symporters located in the small intestine (SGLT1) and kidneys (SGLT2) which exchange sodium and glucose.35,36 The inhibition of SGLT2 in the renal proximal tubule (ie, SGLT2 inhibitors, such as empagliflozin, canagliflozin, dapagliflozin) reduces urinary glucose reuptake, and in doing so, reduces serum glucose levels. 35 These medications have revolutionized pharmacology for type 2 diabetes (T2D), given additional non-glycemic benefits such as cardiac and renal protection. This narrative review seeks to delve into their data in T1D and their potential in HCL.
A Review of the Unmet Needs in Closed-Loop Therapy
Post-Prandial Hyperglycemia
While closed-loop therapy is excellent at reducing hypoglycemia and increasing the safety of intensive insulin therapy, it does not eliminate hyperglycemia. On average, those on HCL spend 5-8 hours per day in hyperglycemia.15,16,37 This is influenced by the carbohydrate load, meal composition, the timing of insulin administration, insulin pharmacokinetics, and gastric emptying. Figure 1 demonstrates insulin-dependent and insulin-independent means to potentially ameliorate hyperglycemia in closed-loop therapy; note SGLTi’s address 2 of them.
Figure 1.
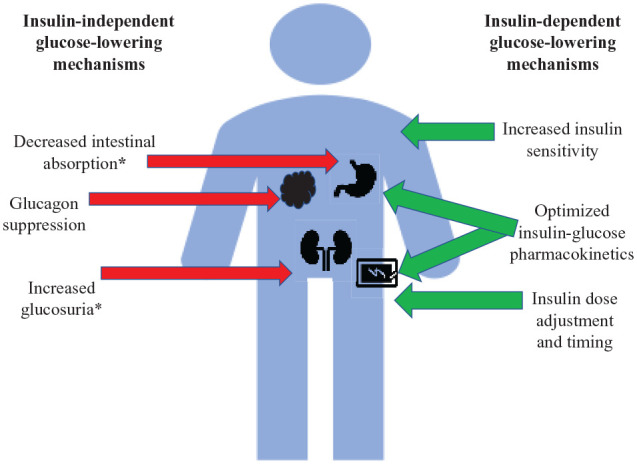
Methods of glucose normalization in type 1 diabetes while on closed-loop therapy.
Items marked with an asterix apply to SGLTi’s mechanism of action.
Carbohydrate Counting
A major barrier to glycemic control in T1D is accurate carbohydrate counting, an essential aspect of insulin dosing.38,39 Prior studies have depicted high rates of counting inaccuracies40,41 and mental health impacts due to disease burden. 42 Carbohydrate counting alleviation could be potentially aided through adjunctive pharmacotherapy, particularly if done through an insulin-independent elimination of glucose.
The Lack of Non-Glycemic Benefits
T1D care includes the reduction of vascular complications and optimization of metabolic health. There is an increasing prevalence of overweight and obese individuals with T1D, with up to 31% higher BMI than the general population.43-45 This results in worsened clinical outcomes; not only is increased BMI in T1D linked with poor glycemic control, 46 but it is further linked with increased cardiac risk factors and complications.46-49
Cardiovascular and kidney disease are significant causes of mortality for T1D.50,51 Though glycemic control improves cardiac and renal outcomes,2,3,52 mortality is still increased in those with well-controlled T1D compared to those without T1D. 53 It is estimated that those with T1D have a life expectancy 11-13 years shorter than those without diabetes of the same age. 54 The treatment of nephropathy in T1D has not seen a breakthrough in over a decade, which includes glycemic and blood pressure control as well as specific pharmacotherapy.2,55-57 Unfortunately, there are not as much data in cardiovascular protection in T1D as there are for renal protection, as T1D is often under-represented in cardiovascular trials. Glycemic control was shown to improve cardiovascular outcomes 17 years later after DCCT, but appears to not be sufficient to completely normalize cardiovascular risk.2,3,58 Adjunct pharmacotherapy may be a way to allow for further metabolic benefits.
SGLTi’s in T1D: A Review of the Randomized Controlled Trials (RCTs) and Real-World Use
A natural interest in SGLTi use sparked in T1D given their novel action and benefit in T2D. Table 1 depicts RCTs assessing SGLTi use in T1D. Canagliflozin was the first drug studied in a major RCT, revealing significant benefits in glycemic control and body weight, while reducing insulin needs without increasing hypoglycemia.59,60 Unfortunately, the rates of diabetic ketoacidosis (DKA) were markedly elevated at 4.3% and 6% with 100 and 300 mg respectively versus none in the placebo group. Following this trial, the DEPICT trials (dapagliflozin), the inTandem trials (using sotagliflozin, an SGLT1 and 2 inhibitor), and the EASE trials (empagliflozin) assessed these agents as adjunct to routine care to T1D management.59,61-69 Various meta-analyses have assessed the collective data on these medications in T1D. The summated reductions are in HbA1c by 0.39%, weight by 3.47%, mean glucose by 1.07 mmol/L, TDD by 10.4%, and systolic blood pressure 3.37 mmHg.70-73 CGM data revealed improvements in TIR without increased hypoglycemia.59,62,64,66,74 Unfortunately, the relative risks of ketoacidosis in those treated with SGLTi compared to placebo were 3-5.70-73
Table 1.
A Summary of Trials in the Use of SGLTi as Adjunct to Insulin Therapy in T1D.
| Study | Doses used | Length of study | Population | HbA1c change from baseline | Total daily dose: change from baseline | Body Weight: change from baseline | Systolic blood pressure: change from baseline | Time in range (3.9-10.0 mmol/L): change from baseline | DKA rates per group |
|---|---|---|---|---|---|---|---|---|---|
| Canagliflozin | |||||||||
| NCT0213994359,60 | 100 mg, 300 mg | 18 weeks | HbA1c 7-9%, aged 25-65, low C-peptide, BMI 21-35 kg/m2 n = 351 | 100 mg: −0.27% 300 mg: −0.24% P: +0.01% | Measured as Units/day: 100 mg: −2.5 300 mg: −6.0 P: +1.6 | As % from baseline 100 mg: −3.1 300 mg: −5.1 P: +0.3 | N/A | 100 mg: +11.7 300 mg: + 10.1 P: −3.5 | 100 mg: 4.3% 300 mg: 6% P: 0% |
| Dapagliflozin | |||||||||
| DEPICT-165,75 | 5 mg, 10 mg | 24 weeks 65 52 weeks 75 NB: 24-week data shown if not clearly stated in 52-week data | HbA1c 7.7-11%, aged 18-75, low C peptide (exclusion if BMI < 18.5, < 0.3 U/kg, recent hypoglycemia or polyuria/polydipsia/ weight loss) DEPICT-1, n = 833 DEPICT-2, n = 814 | P-adjusted at 52 weeks: 5 mg: −0.33% 10 mg: −0.36% | P-adjusted at 24 weeks: 5 mg: −8.8% 10 mg −13.2% | P-adjusted at 52 weeks:5 mg: −2.95% 10 mg: −4.54% | P-adjusted at 52 weeks: 5 mg: −1.12 mmHg (NS) 10 mg: −5.38 mmHg (NS) | At 24 weeks:5 mg: + 7.0 10 mg: + 8.5 P: −2.1% | 5 mg: 4.5% 10 mg: 3.4% P: 1.9% |
| DEPICT-2 68 | 24 weeks 68 52 weeks, as pooled analyses 62 NB: 24-week data shown | P-adjusted: 5 mg: −0.37% 10 mg: −0.42% | P-adjusted: 5 mg: −10.78% 10 mg: −11.08% | P-adjusted: 5 mg: −3.2% 10 mg: −3.74% | N/A | P-adjusted at 24 weeks 5 mg: + 9.0% 10 mg: +10.7% | 5 mg: 2.6% 10 mg:2.2% P: 0% | ||
| DEPICT-5 76 | 52 weeks | Japanese, HbA1c 7.5-10.5%, age 18-75 years old, BMI ≥ 20 kg/m2, low C-peptide, TDD ≥ 0.3 U/kg/day n = 151 | P-adjusted 5 mg: −0.33% 10 mg: −0.36% | P-adjusted, % from baseline 5 mg: −12.27 10 mg: −13.13 | P-adjusted, % from baseline 5 mg: −4.25 10 mg: −5.96 | N/A | N/A | 5 mg: 2.6% (2 patients) 10 mg: 1.3% (1 patient) P: 0% | |
| Sotagliflozin | |||||||||
| InTandem1 66 | 200 mg, 400 mg | 52 weeks | HbA1c 7-11%, >18 years old, BMI >18.5, exclusion if recent severe hypoglycemia or DKA InTandem1, n = 793 patients (North America) InTandem2, n = 782 (Europe and Israel) InTandem3, n = 1402 (world-wide, 133 sites) | P-adjusted 200 mg: −0.36% 400 mg: −0.41% | P-adjusted 200 mg: −8.02% 400 mg: −12.64 % | P-adjusted 200 mg: −3.63% 400 mg: −4.96 % | P-adjusted 200 mg: −2.8 mmHg 400 mg: −4.4 mmHg | 400 mg: +10.4% P: +2.5% | 200 mg: 3.4% 400 mg: 4.2% P: 0.4% |
| InTandem2 74 | 200 mg, 400 mg | 52 weeks | P-adjusted: 200 mg: −0.21% 400 mg: −0.32% | P-adjusted 200 mg: −6.26% 400 mg: −8.17% | P-adjusted 200 mg: −2.78% 400 mg: −3.50% | At 12 weeks 400 mg: −2.8 mmHg (200 mg data not given) | P-adjusted 200 mg: +8.4 400 mg: +13.4 | 200 mg: 2.3% 400 mg: 3.4% P:0% | |
| InTandem3 63 | 400 mg | 24 weeks | P-adjusted: −0.46% | P-adjusted: −9.7% | P-adjusted: −2.98 kg | 400 mg: −3.5 mmHg | N/A | 400 mg: 3% P: 0.6% | |
| InTandem4 69 | 75 mg, 200 mg, 400 mg | 12 weeks | ≥18 years old, HbA1c 7.0-10.0%, GFR > 60 ml/min/1.73 m2 n = 141 | P-adjusted from baseline 75 mg: −0.3 (NS) 200 mg: −0.5 400 mg: −0.4 | P-adjusted, 400 mg: −8.9 Units/day (others not reported, NS) | P-adjusted 75 mg: −1.3 kg 200 mg: −2.4 kg 400 mg: −2.6 kg | P-adjusted 400 mg: −6.1 mmHg (others not reported, NS) | N/A | 400 mg: 1 case (only case in trial) |
| Sotagliflozin JDRF 67 | 400 mg | 12 weeks | Aged 18-30, HbA1c ≥ 9% n = 85 | P-adjusted: −0.4 (NS) | P-adjusted −6.7 (NS) | P-adjusted −2.37 kg | N/A | P-adjusted: 7.7% (NS, P = .057) | 1 subject in 400 mg group only |
| Empagliflozin | |||||||||
| EASE-2,3 (pooled data) 64 | 2.5 mg (EASE-3 only), 10 mg, 25 mg | EASE-2 = 52 weeks EASE-3 = 26 weeks | Low C-peptide, TDD 0.3-1.5 U/kg, BMI ≥ 18.5, GFR ≥ 30 and A1c 7.5-10% after optimization EASE-2 = 723 EASE-3 = 961 | P-adjusted 2.5 mg: −0.28 10 mg: −0.54 25 mg: −0.53 | P-adjusted, % from baseline 2.5 mg: −6.4% 10 mg: −13.3% 25 mg: −12.7% | P-adjusted, in kg 2.5 mg: −1.8 10 mg: −3.0 25 mg: −3.4 | P-adjusted 2.5 g: −2.1 10 mg: −3.9 25 mg: −3.7 | P-adjusted: 2.5 mg: +4.2% (NS) 10 mg: +12.1% 25 mg: +12.9% | 2.5 mg: 0.8% 10 mg: 4.3% 25 mg: 3.3% P: 1.2% |
Note that units of reporting were heterogenous among some trials. (eg, percent change versus change in kilograms for weight).
Abbreviations: N/A, not available; NB, nota bene; NS, not significant; P, placebo.
SGLTi use in T1D has had variable uptake worldwide. In 2019, Europe approved both sotagliflozin and dapagliflozin for T1D.77,78 The National Institute for Health and Care Excellence (NICE) approved the use of dapagliflozin as adjunct to insulin therapy for those with T1D with inadequate control, BMI ≥ 27 kg/m2 and insulin requirements of ≥0.5 Units/kg.77,79 Japan has also approved dapagliflozin and ipragliflozin, a selective SGLT2 inhibitor, for T1D.80,81 In the United States, empagliflozin and sotagliflozin were refused approval by the FDA,82,83 but American off-label use in T1D is still present. 84
SGLT Inhibitor Use and Closed-Loop Therapy: Research thus Far
Though the trials assessing SGLTi’s as adjunct to routine T1D have been extensive, there are only small pilot studies published thus far assessing their potential use in closed-loop therapy (see Table 2). Two studies have used dapagliflozin and empagliflozin as adjunct to closed-loop therapy.85,86 In the study described in Biester et al. 85 dapagliflozin 10 mg BID (2 doses) was used as adjunct to fully closed-loop; participants were given 2 mixed meal tests 6 hours apart without meal announcement. The TIR with dapagliflozin was 68% compared to 50% in the placebo arm (P < .001), and required 22% less insulin.
Table 2.
Prior Studies Assessing the Use of SGLT2i’s as Adjunct to Closed-Loop Therapy.
| Study | Duration per intervention | Number of participants | Study design | Outcomes |
|---|---|---|---|---|
| Biester et al. 85 | 24 hours | 30 (15 adolescents + 15 young adults) | Double-blinded, crossover design, inpatient study: Patient
admission by 4 pm, DreaMed closed-loop therapy by 7 pm,
dapagliflozin 10 mg at night then morning (vs placebo) with 2
unannounced liquid meals, 6 hours apart (ie, fully
automated). Aim: Assess the effect of dapagliflozin on overnight and post prandial glucose on fully automated closed-loop therapy |
TIR was 68 ± 6% using dapagliflozin vs 50 ± 13% in placebo (P < .001) Nocturnal glucose was 6.2 ± 0.7 mmol/L using dapagliflozin vs 7.3 ± 1.7 mmol/L in placebo (P = .003) Total daily dose decreased by 22% (P = .004) No difference in hypoglycemia. Average ketone levels were increased in dapagliflozin vs placebo (0.29 vs 0.16 mmol/L, P < .001) |
| Haidar et al. 86 | 9-14 hours per meal intervention per participant | 30 | Open-label, crossover design: Empagliflozin 25 mg vs placebo with run-in optimization on usual pump therapy x 5- 14 days as outpatient, then trial of closed-loop (McGill Artificial Pancreas) with 3 meal modalities: carbohydrate counting, simple meal announcement, or no meal announcement (full automated). Aim: Assess whether adding empagliflozin 25 mg to closed-loop therapy could reduce the carbohydrate counting burden in T1D without degrading glycemic control | TIR (68 ± 16%) and mean glucose (8.5 ± 1.4 mmol/L) of simple meal announcement + empagliflozin 25 mg were non-inferior to carbohydrate counting + placebo. Empagliflozin 25 mg + carbohydrate counting had the highest TIR (85 ± 11%) and lowest mean glucose (7.4 ± 1.3 mmol/L) of all interventions. Ketone levels were higher in the empagliflozin group (0.22 ± 0.18 mmol/L) than placebo (0.13 ± 0.11 mmol/L, P < .001) No difference in hypoglycemia. |
In Haidar et al. 86 empagliflozin 25 mg daily as adjunct to closed-loop therapy was assessed for alleviation of carbohydrate counting via 3 arms: full carbohydrate counting, simple meal announcement, or no meal announcement. The simple meal announcement with empagliflozin resulted in a daytime mean glucose of 8.5 mmol/L and daytime TIR of 68%, which was non-inferior to full carbohydrate counting alone (TIR 70% with mean glucose 8.5 mmol/L, non-inferiority P = .007). Empagliflozin with full carbohydrate counting resulted in the highest daytime TIR (84%) and lowest mean glucose (7.4 mmol/L), which was significantly lower compared to full carbohydrate counting alone with no empagliflozin (P = .004).
The Benefits of SGLT Inhibitor Use in Closed-Loop Therapy
Glycemic Outcomes and Insulin Dose Optimization
The trials demonstrated in Table 1 revealed decrease in mean fasting glucose, mean glucose throughout the day, and glycemic variability with SGLTi use.70,72,73,87 While improved overnight glucose control is novel in SGLTi use with routine insulin therapy, HCL has already shown superior nocturnal glycemia.18,20,88 Given daytime hyperglycemia is the main obstacle, SGLTi-induced reductions in glucose variability may blunt these rises. The pilot study for sotagliflozin was one of the few studies looking at postprandial effects of SGLTi, where post-breakfast 3-hour CGM data had lower glucose levels compared to placebo. 89 Though postprandial glucose levels were not reported for Haidar et al. (see Table 2), the daytime time-in-range and standard deviation were significantly reduced in the empagliflozin 25 mg group on HCL compared to placebo on HCL. 86 In Biester et al. 85 the areas under the curve for both meals were reduced in the dapagliflozin group compared to placebo, as was standard deviation of glucose.
SGLTi use also reduces the amount of insulin needed to accomplish these glycemic changes. Basal and bolus insulin doses were decreased in the SGLTi trials.72,73 The comparison of basal vs bolus reduction was usually not assessed except for the EASE trials, which found the ratio unchanged. 64 In the small closed-loop studies using SGLT2i’s, the findings were different. In Biester et al. 85 bolus but not basal insulin was significantly reduced in dapagliflozin compared to placebo. In Haidar et al. 86 empagliflozin combined with simple meal announcement on closed-loop had reduced bolus requirements compared to HCL on placebo, yet with non-inferior changes to glycemic control or basal dose. 86 In that same study, empagliflozin with carbohydrate counting resulted in no statistically significant change in mean basal or bolus dose compared to placebo with carbohydrate counting, but TDD was significantly reduced. Though these are small studies, this may suggest insulin utilization is different when SGLTi is combined with closed-loop therapy, and urinary excretion of glucose could bypass the difficulties in insulin pharmacokinetics in HCL.
Alleviation of Carbohydrate Counting
Though commercial HCL may remove a certain amount of nutritional distress, 90 simplified meal announcements and fully closed-loop are the next frontiers in closed-loop insulin delivery systems research.91-95 The main objectives of the studies described in Table 2 were to alleviate carbohydrate counting with the use of closed-loop therapy. While Biester et al demonstrated time-in-range was higher with SGLT2i compared to placebo without meal announcement, Haidar et al. 86 revealed this is still inferior to carbohydrate counting. Adding SGLTi pharmacotherapy to reduce glucose levels while simultaneously optimizing automated insulin delivery through bolus simplification may relieve some of the burden of carbohydrate measurement.
Improved Metabolic Outcomes and Potentially Reductions in Complications
HCL has increased the ability to achieve target glycemic control, which in itself has shown improvements in micro- and macrovascular complications,2,3 but glucose control alone cannot eliminate the increased mortality and vascular complications seen in T1D.53,58 The latest advances in T2D pharmacotherapy, alternatively, have shown advantages in weight management, and renal and cardiovascular protection independent of glucose-lowering properties.96-98
Intensive insulin therapy is known to cause difficulties in weight loss. 99 A comparison of pharmacotherapies in T1D demonstrated SGLTi’s as one of the most effective pharmacotherapies for weight loss.59,63-66,68,100,101 Though SGLTi’s have not yet had an RCT in T1D dedicated to renal outcomes, post-hoc analyses from DEPICT revealed reduction in albuminuria with dapagliflozin; 102 a meta-analysis also revealed benefits with sotagliflozin. 103 A study using empagliflozin 25 mg for 8 weeks in those with T1D demonstrated that in clamped euglycemia, renal hyperfiltration (an early sign of diabetic nephropathy) was attenuated by the use of empagliflozin. 104 In the trial DAPA-CKD where dapagliflozin was administered to participants with chronic kidney disease, of whom 32% did not have diabetes, reductions in progression of chronic kidney disease were seen in those taking dapagliflozin, independent of diabetes diagnosis. 105 Because benefits were seen without diabetes, their mechanism of action may then be also protective in those with T1D.
In the EMPEROR and DAPA-HF trials, cardiac outcomes were assessed in those with heart failure, with or without T2D, after using empagliflozin and dapagliflozin respectively, which showed cardio-protective benefits regardless of diabetes diagnosis.106,107 Unfortunately, EMPEROR excluded those with any history of ketoacidosis, while DAPA-HF excluded those with T1D altogether. Heart failure, whether it be due to preserved or reduced ejection fraction, is often underdiagnosed with individuals with T1D. 108 SGLT2i's may at least improve the cardiovascular risk of hypertension; many of the studies of SGLTi as adjunct in T1D showed blood pressure reductions (Table 1). Though SGLTi use may slightly increase both LDL and HDL cholesterols, this was not linked with increased cardiac risk.96,109
Potential Risks of SGLT Inhibitor Use in Closed-Loop Therapy
The main barrier to implementation of SGLTi use in T1D care is the fear of DKA. It is a common concern in T1D; in the T1D Exchange, 2%-7% of adults with T1D had an episode of DKA in 2016. 110 This is particularly concerning because RCT data often underestimates the complications eventually seen in real-world data, as what was seen with DKA with SGLT2i use in T2D.111-114
The studies assessing SGLTi use in closed-loop therapy were too small to assess DKA risk but demonstrate slight but significant elevations in ketone levels.85,86 A case report of DKA with SGLTi while on HCL has previously been published, 115 which highlights the risks of ketoacidosis during reduced insulin delivery and decreased patient oversight when a pump’s algorithm is fully trusted. Though these risks differ from non-automated pump therapy, the known risks on routine pump therapy, such as catheter malfunctions, are still present. Many of the alarms on commercial systems to warn of ketone risk are geared towards hyperglycemia, which do not acknowledge euglycemic ketoacidosis with SGLTi use.
So why the increased risk? Increased urinary glucose excretion reduces insulin requirements, increasing lipolysis and fatty acid delivery to the liver, as well as increasing glucagon secretion, thus switching energy use to ketosis.116-118 In a study where markers of lipolysis were measured during insulin withdrawal in those with T1D on dapagliflozin vs placebo, the increase in lipolysis markers were higher in those taking dapagliflozin compared to the expected rise in placebo. 119
Further subanalyses pertaining to DKA were performed within the large studies assessing SGLTi use in T1D. As the first large trial, the canagliflozin study originally lacked strategic DKA prevention strategies. 59 All cases were linked to known precipitants of DKA such as acute illness or catheter malfunctions without differences in baseline characteristics from the study population. 120 For InTandem-1,2, the exposure-adjusted incidence rates (EAIRs) for DKA were 3.1 events per 100 person-years for sotagliflozin 200 mg and 4.2 per 100 person-years for sotagliflozin 400 mg, in comparison to 0.2 for placebo. Larger reductions in TDD were associated with increased risk of DKA. Those who developed DKA on sotagliflozin were (for sotagliflozin 400 mg): more likely to be female (EAIR 6.2), on pump therapy (EAIR 6.0), with TDD < 0.7 IU/kg (EAIR 4.9), and body mass index (BMI) < 27 kg/m2 (EAIR 4.4). Higher BMI may be protective due to lower rates of lipolysis compared to lean individuals.121,122 A detailed risk mitigation plan initiated later on in InTandem-1 and -2 allowed for reductions in DKA incidence rates. 123
Risk factors similar to InTandem were seen in the EASE trials, where of the 72 cases of DKA, 53 were in women, and 48 were with pump therapy. 64 The increased risk of DKA in women is seen independent of SGLT2i use; in the T1D exchange, DKA rates were higher in women. 110 This could be related to an increased rate of lipolysis in women. 124
Going Forward: Research and Clinical Strategies to Optimize SGLTi Use in HCL
There are potential benefits and risks by adding SGLTi pharmacotherapy to HCL. Given the small studies, more research must be done. Though empagliflozin 2.5 mg’s effect on CGM did not reach significance in EASE-3, 64 its potential may be synergistic with HCL and enough to reduce the post-prandial excursions seen in HCL. Further studies are underway to assess empagliflozin’s utility in HCL (NCT04450563, NCT03979352, NCT04201496). Studies of larger sample sizes and longer duration will aid in assessing therapeutic effects, specifically (1) degree of glycemic improvement, particularly in those who do not achieve goals on HCL, (2) what aspect of HCL is modified (ie, bolus vs basal changes, prandial vs fasting glucose), and (3) the risks associated with this regimen. Further observational studies in commercial HCL systems will also provide guidance.
Risk mitigation must be accomplished prior to widespread use of this regimen. This involves revision of clinical strategies and appropriate patient selection. While prior recommendations have described the targeted population for this therapy,125,126 Table 3 expands on these concepts to HCL and other potential benefits.
Table 3.
A Consideration of Patient Choice in Strategic SGLTi Use as Adjunct to Closed-Loop Therapy in Those with Type 1 Diabetes.
| Suggested indications for use |
Those to avoid prescribing or do so
cautiously |
||
|---|---|---|---|
| Validated | To be investigated | Avoid | Caution |
| Overweight/obese patients (in particular BMI > 27 kg/m2) | Post-prandial elevations despite closed-loop insulin therapy | Non-compliant with ketone protocol | CSII use with frequent catheter malfunctions |
| Insulin resistance (>0.5 Units/kg) | Cardiovascular disease | Recurrent DKA and other ketosis events, or recent DKA (within 12 months) | Young women |
| Metabolic syndrome | Relief in precise carbohydrate counting | Ketogenic or very low calorie diet | TDD < 0.5 Units/kg |
| Hyperglycemia despite optimized insulin therapy | Inability to achieve TIR > 70% | Severe non-compliance or deficits in diabetes self-management | BMI < 25 kg/m2 |
| Desire for blood pressure control along with achieving euglycemia | BMI ≤ 18.5 kg/m2 | ||
| Albuminuria and diabetic nephropathy (emerging data) | Recurrent serious infections or acute illness (eg, osteomyelitis, cellulitis, urosepsis, foot ulcers) | ||
| Total daily dose <0.3 U/kg/day | |||
| Age < 18 or >75 years old | |||
The most important method to reduce risk of DKA is ketone monitoring. Very recently a continuous ketone monitor device has been proposed. 127 Ideally, the device’s ketone data could be integrated into the insulin-dosing algorithm to increase insulin requirements in order to reduce ketone levels in an additional closed-loop fashion. Until this is feasible, clinical and technological strategies are required. The aforementioned risk factors are incorporated into various guidelines to mitigate DKA risk in SGLT2i use in T1D.126,128 Both the STICH and STOP-DKA are protocols that describe approaches to mitigating DKA risk and reducing ketone levels with SGLTi use in T1D.125,129 Unfortunately, these do not address HCL. To avoid the risk of DKA, not only from routine pump malfunction but from possible minimal or suspended insulin delivery, clear recommendations must be given. These include recommended carbohydrate intake, additional insulin bolus doses, or potentially, turning off the algorithm in order to manually increase insulin delivery.
Conclusions
As it emerges into commercial use, HCL is becoming a life-changing therapy and may eventually dominate insulin pump therapy as CGM becomes standard-of-care. As those with T1D move onto closed-loop therapy, they may want to bring their pre-existing SGLTi prescriptions with them. Those who transition to HCL may also find themselves with difficulties in reducing post-prandial hyperglycemia, or struggling with weight loss or vascular complications. The use of SGLTi could optimize HCL, and would efficiently add the benefits of weight loss, blood pressure control, and renoprotection that HCL alone cannot offer. The use of SGLTi in T1D remains a controversial topic, yet there are significant potentials for closing the gaps in routine care.
Acknowledgments
None
Footnotes
Abbreviations: BMI, body mass index; CGM, continuous glucose monitoring; DKA, diabetic ketoacidosis; EAIR, exposure adjusted incidence rates; HbA1c, glycated hemoglobin; HCL, hybrid closed-loop therapy; RCT, randomized controlled trial; SGLT(i), sodium-glucose linked transporter (inhibitors); T1D, type 1 diabetes; T2D, type 2 diabetes; TDD, total daily dose; TIR, time in target range.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: A.H. received research support/consulting fees from Eli Lilly, Medtronic, Aga- Matrix, Adocia, and Dexcom and has pending patents in the artificial pancreas area. M.A.T. received re- search support from AgaMatrix, consulting fees from Sanofi and Adocia, and speaker honoraria from Eli Lilly, Novo Nordisk, Boehringer Ingelheim, Janssen, and AstraZeneca. No other potential conflicts of interest relevant to this article were reported.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Melissa-Rosina Pasqua  https://orcid.org/0000-0003-2389-9066
https://orcid.org/0000-0003-2389-9066
References
- 1. Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. [DOI] [PubMed] [Google Scholar]
- 2. The Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977-986. [DOI] [PubMed] [Google Scholar]
- 3. Nathan DM, Cleary PA, Backlund JY, et al. ; The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Diabetes Canada Clinical Practice Guidelines Expert Committee; Imran SA, Agarwal G, Bajaj HS, Ross S. Targets for glycemic control. Can J Diabetes. 2018;42(suppl 1):S42-S46. [DOI] [PubMed] [Google Scholar]
- 5. American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(suppl 1):S66-S76. [DOI] [PubMed] [Google Scholar]
- 6. Matuleviciene V, Attvall S, Ekelund M, et al. A retrospective study in 5,989 patients with type 1 diabetes in 10 outpatient diabetes clinics in Sweden of the frequency of measuring HbA1c in clinical practice. J Diabetes Metab. 2014;5(5):1-7. [Google Scholar]
- 7. Miller KM, Foster NC, Beck RW, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D exchange clinic registry. Diabetes Care. 2015;38(6):971-978. [DOI] [PubMed] [Google Scholar]
- 8. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016-2018. Diabetes Technol Ther. 2019;21(2):66-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shah VN, Grimsmann JM, Foster NC, et al. Undertreatment of cardiovascular risk factors in the type 1 diabetes exchange clinic network (United States) and the prospective diabetes follow-up (Germany/Austria) registries. Diabetes Obes Metab. 2020;22:1577-1585. [DOI] [PubMed] [Google Scholar]
- 10. Aronson R, Brown RE, Abitbol A, et al. The Canadian LMC diabetes registry: a profile of the demographics, management, and outcomes of individuals with type 1 diabetes. Diabetes Technol Ther. 2020;23(1):31-40. [DOI] [PubMed] [Google Scholar]
- 11. Senior P, Lam A, Farnsworth K, Perkins B, Rabasa-Lhoret R. Assessment of risks and benefits of beta cell replacement versus automated insulin delivery systems for type 1 diabetes. Curr Diab Rep. 2020;20(10):52. [DOI] [PubMed] [Google Scholar]
- 12. Accili D. Whither type 1 diabetes? N Engl J Med. 2020;383(21):2078-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tubiana-Rufi N, Schaepelynck P, Franc S, et al. Practical implementation of automated closed-loop insulin delivery: a French position statement. Diabetes Metab. 2021;47(3):101206. [DOI] [PubMed] [Google Scholar]
- 14. Boughton CK, Hovorka R. New closed-loop insulin systems. Diabetologia. 2021;64(5):1007-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thabit H, Tauschmann M, Allen JM, Leelarathna L, Hartnell S, Wilinska ME. Home use of an artificial beta cell in type 1 diabetes. N Engl J Med. 2015;373(22):2129-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patientswith type 1 diabetes. JAMA. 2016;316(13):1407-1408. [DOI] [PubMed] [Google Scholar]
- 17. Haidar A, Legault L, Messier V, Mitre TM, Leroux C, Rabasa-Lhoret R. Comparison of dual-hormone artificial pancreas, single-hormone artificial pancreas, and conventional insulin pump therapy for glycaemic control in patients with type 1 diabetes: an open-label randomised controlled crossover trial. Lancet Diabetes Endocrinol. 2015;3(1):17-26. [DOI] [PubMed] [Google Scholar]
- 18. Haidar A, Legault L, Matteau-Pelletier L, et al. Outpatient overnight glucose control with dual-hormone artificial pancreas, single-hormone artificial pancreas, or conventional insulin pump therapy in children and adolescents with type 1 diabetes: an open-label, randomised controlled trial. Lancet Diabetes Endocrinol. 2015;3(8):595-604. [DOI] [PubMed] [Google Scholar]
- 19. Haidar A, Tsoukas MA, Bernier-Twardy S, et al. A novel dual-hormone insulin-and-pramlintide artificial pancreas for type 1 diabetes: a randomized controlled crossover trial. Diabetes Care. 2020;43(3):597-606. [DOI] [PubMed] [Google Scholar]
- 20. Brown SA, Kovatchev BP, Raghinaru D, et al. Six month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med. 2019;381(18):1707-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Forlenza GP, Pinhas-Hamiel O, Liljenquist DR, et al. Safety evaluation of the minimed 670g system in children 7-13 years of age with type 1 diabetes. Diabetes Technol Ther. 2019;21(1):11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Levetan C, Want LL, Weyer C, et al. Impact of pramlintide on glucose fluctuations and postprandial glucose, glucagon, and triglyceride excursions among patients with type 1 diabetes intensively treated with insulin pumps. Diabetes Care. 2003;26(1):1-8. [DOI] [PubMed] [Google Scholar]
- 23. Weinzimer SA, Sherr JL, Cengiz E, et al. Effect of pramlintide on prandial glycemic excursions during closed-loop control in adolescents and young adults with type 1 diabetes. Diabetes Care. 2012;35(10):1994-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Renukuntla VS, Ramchandani N, Trast J, Cantwell M, Heptulla RA. Role of Glucagon-like peptide-1 analogue versus Amylin as an adjuvant therapy in type 1 diabetes in a closed loop setting with ePID algorithm. J Diabetes Sci Technol. 2014;8(5):1011-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sherr JL, Patel NS, Michaud CI, et al. Mitigating meal-related glycemic excursions in an insulin-sparing manner during closed-loop insulin delivery: the beneficial effects of adjunctive pramlintide and liraglutide. Diabetes Care. 2016;39(7):1127-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ilkowitz JT, Katikaneni R, Cantwell M, Ramchandani N, Heptulla RA. Adjuvant liraglutide and insulin versus insulin monotherapy in the closed-loop system in type 1 diabetes: a randomized open-labeled crossover design trial. J Diabetes Sci Technol. 2016;10(5):1108-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Underland LJ, Ilkowitz JT, Katikaneni R, Dowd A, Heptulla RA. Use of sitagliptin with closed-loop technology to decrease postprandial blood glucose in type 1 diabetes. J Diabetes Sci Technol. 2017;11(3):602-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wilson LM, Jacobs PG, Ramsey KL, et al. Dual-hormone closed-loop system using a liquid stable glucagon formulation versus insulin-only closed-loop system compared with a predictive low glucose suspend system: an open-label, outpatient, single-center, crossover, randomized controlled trial. Diabetes Care. 2020;43(11):2721-2729. [DOI] [PubMed] [Google Scholar]
- 29. Langford BE, Evans M, Haskins-Coulter T, et al. Systematic literature review and network meta-analysis of sodium-glucose co-transporter inhibitors vs metformin as add-on to insulin in type 1 diabetes. Diabetes Obes Metab. 2020;22(1):39-50. [DOI] [PubMed] [Google Scholar]
- 30. Mathieu C, Zinman B, Hemmingsson JU, et al. Efficacy and safety of liraglutide added to insulin treatment in type 1 diabetes: the adjunct one treat-to-target randomized trial. Diabetes Care. 2016;39(10):1702-1710. [DOI] [PubMed] [Google Scholar]
- 31. Ahren B, Hirsch IB, Pieber TR, et al. Efficacy and safety of liraglutide added to capped insulin treatment in subjects with type 1 diabetes: the adjunct two randomized trial. Diabetes Care. 2016;39(10):1693-1701. [DOI] [PubMed] [Google Scholar]
- 32. Dejgaard TF, Schmidt S, Frandsen CS, et al. Liraglutide reduces hyperglycaemia and body weight in overweight, dysregulated insulin-pump-treated patients with type 1 diabetes: the Lira Pump trial—a randomized, double-blinded, placebo-controlled trial. Diabetes Obes Metab. 2020;22(4):492-500. [DOI] [PubMed] [Google Scholar]
- 33. Johansen NJ, Dejgaard TF, Lund A, et al. Efficacy and safety of meal-time administration of short-acting exenatide for glycaemic control in type 1 diabetes (MAG1C): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2020;8(4):313-324. [DOI] [PubMed] [Google Scholar]
- 34. Avgerinos I, Karagiannis T, Matthews DR, Liakos A. Comparative efficacy and safety of glucose-lowering drugs as adjunctive therapy for adults with type 1 diabetes : a systematic review and network meta-analysis. Diabetes Obes Metab. 2020;1-10. [DOI] [PubMed] [Google Scholar]
- 35. Tahrani AA, Barnett AH, Bailey CJ. SGLT inhibitors in management of diabetes. Lancet Diabetes Endocrinol. 2013;1(2):140-151. [DOI] [PubMed] [Google Scholar]
- 36. Wright EM, Hirayama BA, Loo DF. Active sugar transport in health and disease. J Intern Med. 2007;261(1):32-43. [DOI] [PubMed] [Google Scholar]
- 37. Gingras V, Taleb N, Roy-Fleming A, Legault L, Rabasa-Lhoret R. The challenges of achieving postprandial glucose control using closed-loop systems in patients with type 1 diabetes. Diabetes Obes Metab. 2018;20:245-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sievenpiper JL, Chan CB, Dworatzek PD, Freeze C, Williams SL. Nutrition therapy 2018 clinical practice guidelines. Can J Diabetes. 2018;42:S64-S79. [DOI] [PubMed] [Google Scholar]
- 39. Evert AB, Boucher JL, Cypress M, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2014;37(suppl 1):120-143. [DOI] [PubMed] [Google Scholar]
- 40. Bishop FK, Maahs DM, Spiegel G, et al. The carbohydrate counting in adolescents with type 1 diabetes (CCAT) study. Diabetes Spectr. 2009;22(1):56-62. [Google Scholar]
- 41. Spiegel G, Bortsov A, Bishop FK, et al. Randomized nutrition education intervention to improve carbohydrate counting in adolescents with type 1 diabetes study: is more intensive education needed? J Acad Nutr Diet. 2012;112(11):1736-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lancaster BM, Pfeffer B, McElligott M, et al. Assessing treatment barriers in young adults with type 1 diabetes. Diabetes Res Clin Pract. 2010;90(3):243-249. [DOI] [PubMed] [Google Scholar]
- 43. Minges KE, Whittemore R, Weinzimer SA, Irwin ML, Redeker NS, Grey M. Correlates of overweight and obesity in 5529 adolescents with type 1 diabetes: the T1D exchange clinic registry. Diabetes Res Clin Pract. 2017;126:68-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Baskaran C, Section E, Place OJ, et al. A decade of temporal trends in overweight/obesity in youth with type 1 diabetes after the diabetes control and complications trial (DCCT). Pediatr Diabetes. 2015;16(4):263-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu LL, Lawrence JM, Davis C, et al. Prevalence of overweight and obesity in youth with diabetes in USA: the SEARCH for diabetes in youth study. Pediatr Diabetes. 2010;11(1):4-11. [DOI] [PubMed] [Google Scholar]
- 46. Melin EO, Thulesius HO, Hillman M, Landin-Olsson M, Thunander M. Abdominal obesity in type 1 diabetes associated with gender, cardiovascular risk factors and complications, and difficulties achieving treatment targets: a cross sectional study at a secondary care diabetes clinic. BMC Obes. 2018;5(1):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. De Block CEM, De Leeuw IH, Van Gaal LF. Impact of overweight on chronic microvascular complications in type 1 diabetic patients. Diabetes Care. 2005;28(7):1649-1655. [DOI] [PubMed] [Google Scholar]
- 48. Price SA, Gorelik A, Fourlanos S, Colman PG, Wentworth JM. Obesity is associated with retinopathy and macrovascular disease in type 1 diabetes. Obes Res Clin Pract. 2014;8(2):e178-e182. [DOI] [PubMed] [Google Scholar]
- 49. Couper JJ, Jones TW, Chee M, et al. Determinants of cardiovascular risk in 7,000 youth with type 1 diabetes in the Australasian Diabetes Data Network. J Clin Endocrinol Metab. 2020;106(1):133-142. [DOI] [PubMed] [Google Scholar]
- 50. Secrest AM, Washington RE, Orchard TJ. Chapter 35: mortality in type 1 diabetes. In: Cowie CC, Casagrande SS, Menke A, et al. , eds. Diabetes in America. 3rd ed. National Institute of Diabetes and Digestive and Kidney Diseases (US); 2014:1-16. [Google Scholar]
- 51. Groop PH, Thomas MC, Moran JL, et al. The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes. 2009;58(7):1651-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang PH, Lau J, Chalmers TC. Meta-analysis of effects of intensive blood-glucose control on late complications of type I diabetes. Lancet. 1993;341(8856):1306-1309. [DOI] [PubMed] [Google Scholar]
- 53. Lind M, Svensson A-M, Kosiborod M, et al. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med. 2014;371(21):1972-1982. [DOI] [PubMed] [Google Scholar]
- 54. Livingstone SJ, Levin D, Looker HC, et al. Estimated life expectancy in a scottish cohort with type 1 diabetes, 2008-2010. JAMA. 2015;313(1):37-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lewis JB, Berl T, Bain RP, Rohde RD, Lewis EJ. Effect of intensive blood pressure control on the course of type 1 diabetic nephropathy. Am J Kidney Dis. 1999;34(5):809-817. [DOI] [PubMed] [Google Scholar]
- 56. Andersen S, Tarnow L, Rossing P, Hansen BV, Parving HH. Renoprotective effects of angiotensin II receptor blockade in type 1 diabetic patients with diabetic nephropathy. Kidney Int. 2000;57(2):601-606. [DOI] [PubMed] [Google Scholar]
- 57. Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med. 1993;329(20):1456-1462. [DOI] [PubMed] [Google Scholar]
- 58. Bebu I, Braffett BH, Orchard TJ, Lorenzi GM, Lachin JM. Mediation of the effect of glycemia on the risk of CVD outcomes in type 1 diabetes: the DCCT/EDIC study. Diabetes Care. 2019;42(7):1284-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Henry RR, Thakkar P, Tong C, Polidori D, Alba M. Efficacy and safety of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to insulin in patients with type 1 diabetes. Diabetes Care. 2015;38(12):2258-2265. [DOI] [PubMed] [Google Scholar]
- 60. Rodbard HW, Peters AL, Slee A, Cao A, Traina SB, Alba M. The effect of canagliflozin, a sodium glucose cotransporter 2 inhibitor, on glycemic end points assessed by continuous glucose monitoring and patient-reported outcomes among people with type 1 diabetes. Diabetes Care. 2017;40(2):171-180. [DOI] [PubMed] [Google Scholar]
- 61. Argento NB, Nakamura K. Glycemic effects of SGLT-2 Inhibitor Canagliflozin in type 1 diabetes patients using the dexcom G4 platinum CGM. Endocr Pract. 2016;22(3):315-322. [DOI] [PubMed] [Google Scholar]
- 62. Phillip M, Mathieu C, Lind M, et al. Long-term efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes: pooled 52-week outcomes from the DEPICT-1 and -2 studies. Diabetes Obes Metab. 2021;23(2):549-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Garg SK, Henry RR, Banks P, et al. Effects of sotagliflozin added to insulin in patients with type 1 diabetes. N Engl J Med. 2017;377(24):2337-2348. [DOI] [PubMed] [Google Scholar]
- 64. Rosenstock J, Marquard J, Laffel LM, et al. Empagliflozin as adjunctive to insulin therapyin type 1 diabetes: the EASE trials. Diabetes Care. 2018;41(12):2560-2569. [DOI] [PubMed] [Google Scholar]
- 65. Dandona P, Mathieu C, Phillip M, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (DEPICT-1): 24 week results from a multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5(11):864-876. [DOI] [PubMed] [Google Scholar]
- 66. Buse JB, Garg SK, Rosenstock J, et al. Sotagliflozin in combination with optimized insulin therapy in adults with type 1 diabetes: the North American in Tandem1 study. Diabetes Care. 2018;41(9):1970-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bode BW, Cengiz E, Wadwa RP, et al. Effects of sotagliflozin combined with intensive insulin therapy in young adults with poorly controlled type 1 diabetes: the JDRF sotagliflozin study. Diabetes Technol Ther. 2020;23(1):59-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mathieu C, Dandona P, Gillard P, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (the DEPICT-2 study): 24-week results from a randomized controlled trial. Diabetes Care. 2018;41(9):1938-1946. [DOI] [PubMed] [Google Scholar]
- 69. Baker C, Wason S, Banks P, et al. Dose-dependent glycometabolic effects of sotagliflozin on type 1 diabetes over 12 weeks: the inTandem4 trial. Diabetes Obes Metab. 2019;21(11):2440-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yamada T, Shojima N, Noma H, Yamauchi T, Kadowaki T. Sodium-glucose co-transporter-2 inhibitors as add-on therapy to insulin for type 1 diabetes mellitus: systematic review and meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2018;20(7):1755-1761. [DOI] [PubMed] [Google Scholar]
- 71. El Masri D, Ghosh S, Jaber LA. Safety and efficacy of sodium-glucose cotransporter 2 (SGLT2) inhibitors in type 1 diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2018;137:83-92. [DOI] [PubMed] [Google Scholar]
- 72. Li K, Xu G. Safety and efficacy of sodium glucose co-transporter 2 inhibitors combined with insulin in adults with type 1 diabetes: a meta-analysis of randomized controlled trials. J Diabetes. 2019;11(8):645-655. [DOI] [PubMed] [Google Scholar]
- 73. Zou H, Liu L, Guo J, et al. Sodium–glucose cotransporter inhibitors as add-on therapy in addition to insulin for type 1 diabetes mellitus: a meta-analysis of randomized controlled trials. J Diabetes Investig. 2021;12(4):546-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Danne T, Joish VN, Afonso M, et al. Improvement in patient-reported outcomes in adults with type 1 diabetes treated with sotagliflozin plus insulin vs. insulin alone. Diabetes Technol Ther. 2020;23(1):70-77. [DOI] [PubMed] [Google Scholar]
- 75. Dandona P, Mathieu C, Phillip M, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes: the DEPICT-1 52-week study. Diabetes Care. 2018;41(12):2552-2559. [DOI] [PubMed] [Google Scholar]
- 76. Araki E, Watada H, Uchigata Y, et al. Efficacy and safety of dapagliflozin in Japanese patients with inadequately controlled type 1 diabetes (DEPICT-5): 52-week results from a randomized, open-label, phase III clinical trial. Diabetes Obes Metab. 2020;22(4):540-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kemp A. Forxiga Aproved in Europe in Type-1 Diabetes [Internet]. AstraZeneca PLC; 2019. Accessed December 30, 2020. https://www.astrazeneca.com/media-centre/press-releases/2019/forxiga-approved-in-europe-for-type-1-diabetes22032019.html#:~:text=The European Commission (EC) has,control despite optimal insulin therapy [Google Scholar]
- 78. Sanofi LP. ZynquistaTM Now Approved in the European Union for Treatment of Adults with Type 1 Diabetes. [Internet]. Paris, France and The Woodlands; 2019. Accessed December 30, 2020. https://www.sanofi.com/en/media-room/press-releases/2019/2019-04-26-22-00-00 [Google Scholar]
- 79. National Institute for Health and Care Excellence. Dapagliflozin with Insulin for Treating Type 1 Diabetes [Internet]. NICE; 2019:1-24. Accessed January 26, 2020. https://www.nice.org.uk/guidance/ta597/resources/dapagliflozin-with-insulin-for-treating-type-1-diabetes-pdf-82607268776389 [Google Scholar]
- 80. Kemp A. Forxiga Approved in Japan for Type 1 Diabetes [Internet]. AstraZeneca PLC; 2019. Accessed December 30, 2020. https://www.astrazeneca.com/media-centre/press-releases/2019/forxiga-approved-in-japan-for-type-1-diabetes-27032019.html [Google Scholar]
- 81. Astellas Pharma. Approval of Suglat tablets, selective SGLT2 inhibitor, for additional indication of type 1 diabetes mellitus and additional dosage and administration in Japan. Press Release [Internet]. 2018. [Google Scholar]
- 82. Food and Drug Administration. FDA Briefing Document PRALUENT Endocrinologic and Metabolic Drugs Advisory Committee Meeting. FDA. [Google Scholar]
- 83. Tucker M. FDA Turns Down Sotagliflozin for Type 1 Diabetes [Internet]. Medscape; 2019. Accessed December 30, 2020. https://www.medscape.com/viewarticle/921303 [Google Scholar]
- 84. Hampp C, Swain RS, Horgan C, et al. Use of sodium-glucose cotransporter 2 inhibitors in patients with type 1 diabetes and rates of diabetic ketoacidosis. Diabetes Care. 2020;43(1):90-97. [DOI] [PubMed] [Google Scholar]
- 85. Biester T, Muller I, von dem Berge T, et al. Add-on therapy with dapagliflozin under full closed loop control improves time in range in adolescents and young adults with type 1 diabetes: the DAPADream study. Diabetes Obes Metab. 2021;23(2):599-608. [DOI] [PubMed] [Google Scholar]
- 86. Haidar A, Yale JF, Lovblom LE, et al. Reducing the need for carbohydrate counting in type 1 diabetes using closed- loop automated insulin delivery (artificial pancreas) and empagliflozin: a randomised controlled non-inferiority crossover pilot trial. Diabetes Obes Metab. 2021;23(6):1272-1281. [DOI] [PubMed] [Google Scholar]
- 87. Lu J, Tang L, Meng H, Zhao J, Liang Y. Effects of sodium-glucose cotransporter (SGLT) inhibitors in addition to insulin therapy on glucose control and safety outcomes in adults with type 1 diabetes: a meta-analysis of randomized controlled trials. Diabetes Metab Res Rev. 2019;35(7):1-10. [DOI] [PubMed] [Google Scholar]
- 88. Bekiari E, Kitsios K, Thabit H, et al. Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis. BMJ. 2018;361:k1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sands AT, Zambrowicz BP, Rosenstock J, et al. Sotagliflozin, a dual SGLT1 and SGLT2 inhibitor, as adjunct therapy to insulin in type 1 diabetes. Diabetes Care. 2015;38(7):1181-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lawton J, Blackburn M, Rankin D, et al. The impact of using a closed-loop system on food choices and eating practices among people with Type 1 diabetes: a qualitative study involving adults, teenagers and parents. Diabet Med. 2019;36(6):753-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gingras V, Haidar A, Messier V, Legault L, Ladouceur M, Rabasa-Lhoret R. A simplified semiquantitative meal bolus strategy combined with single- and dual-hormone closed-loop delivery in patients with type 1 diabetes: a pilot study. Diabetes Technol Ther. 2016;18(8):464-471. [DOI] [PubMed] [Google Scholar]
- 92. Gingras V, Rabasa-Lhoret R, Messier V, Ladouceur M, Legault L, Haidar A. Efficacy of dual-hormone artificial pancreas to alleviate the carbohydrate-counting burden of type 1 diabetes: a randomized crossover trial. Diabetes Metab. 2016;42(1):47-54. [DOI] [PubMed] [Google Scholar]
- 93. Cameron FM, Ly TT, Buckingham BA, et al. Closed-loop control without meal announcement in type 1 diabetes. Diabetes Technol Ther. 2017;19(9):527-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Song L, Liu C, Yang W, et al. Glucose outcomes of a learning-type artificial pancreas with an unannounced meal in type 1 diabetes. Comput Methods Programs Biomed. 2020;191:105416. [DOI] [PubMed] [Google Scholar]
- 95. Palisaitis E, El Fathi A, von Oettingen JE, Haidar A, Legault L. A meal detection algorithm for the artificial pancreas: a randomized controlled clinical trial in adolescents with type 1 diabetes. Diabetes Care. 2021;44(2):604-606. [DOI] [PubMed] [Google Scholar]
- 96. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. [DOI] [PubMed] [Google Scholar]
- 97. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-306. [DOI] [PubMed] [Google Scholar]
- 98. Senior PA, Houlden RL, Kim J, et al. Pharmacologic glycemic management of type 2 diabetes in adults: 2020 update – the user’s guide. Can J Diabetes. 2020;44(7):592-596. [DOI] [PubMed] [Google Scholar]
- 99. Wing RR, Cleary PA. Weight gain associated with intensive therapy in the diabetes control and complications trial. Diabetes Care. 1988;11(7):567-573. [DOI] [PubMed] [Google Scholar]
- 100. Tandon S, Ayis S, Hopkins D, Harding S, Stadler M. The impact of pharmacological and lifestyle interventions on body weight in people with type 1 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2021;23(2):350-362. [DOI] [PubMed] [Google Scholar]
- 101. Danne T, Cariou B, Banks P, et al. HbA1c and hypoglycemia reductions at 24 and 52 weeks with sotagliflozin in combination with insulin in adults with type 1 diabetes: the European in Tandem2 study. Diabetes Care. 2018;41(9):1981-1990. [DOI] [PubMed] [Google Scholar]
- 102. Groop PH, Dandona P, Phillip M, et al. Effect of dapagliflozin as an adjunct to insulin over 52 weeks in individuals with type 1 diabetes: post-hoc renal analysis of the DEPICT randomised controlled trials. Lancet Diabetes Endocrinol. 2020;8(10):845-854. [DOI] [PubMed] [Google Scholar]
- 103. Musso G, Gambino R, Cassader M, Paschetta E. Efficacy and safety of dual SGLT 1/2 inhibitor sotagliflozin in type 1 diabetes: meta-analysis of randomised controlled trials. BMJ. 2019;365:l1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Cherney DZI, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129(5):587-597. [DOI] [PubMed] [Google Scholar]
- 105. Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436-1446. [DOI] [PubMed] [Google Scholar]
- 106. Packer M, Butler AJ, Filippatos G, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413-1424. [DOI] [PubMed] [Google Scholar]
- 107. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995-2008. [DOI] [PubMed] [Google Scholar]
- 108. McAllister DA, Read SH, Kerssens J, et al. Incidence of hospitalization for heart failure and case-fatality among 3.25 million people with and without diabetes mellitus. Circulation. 2018;138(24):2774-2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644-657. [DOI] [PubMed] [Google Scholar]
- 110. Shah VN, Wu M, Polsky S, et al. Gender differences in diabetes self-care in adults with type 1 diabetes: findings from the T1D exchange clinic registry. J Diabetes Complications. 2018;32(10):961-965. [DOI] [PubMed] [Google Scholar]
- 111. U.S. Food and Drug Administration. FDA Revises Labels of SGLT2 Inhibitors for Diabetes to Include Warnings About Too Much Acid in the Blood and Serious Urinary Tract Infections. FDA; 2020:1-8. [Google Scholar]
- 112. Government of Canada. Summary Safety Review - SGLT2 Inhibitors (Canagliflozin, Dapagliflozin, Empagliflozin) - Assessing the Risk of the Body Producing High Levels of Acids in the Blood (Diabetic Ketoacidosis). Government of Canada; 2016. [Google Scholar]
- 113. Goldenberg RM, Berard LD, Cheng AYY, et al. SGLT2 inhibitor–associated diabetic ketoacidosis: clinical review and recommendations for prevention and diagnosis. Clin Ther. 2016;38(12):2654-2664.e1. [DOI] [PubMed] [Google Scholar]
- 114. Handelsman Y, Henry RR, Bloomgarden ZT, et al. American association of clinical endocrinologists and American college of endocrinology position statement on the association of SGLT-2 inhibitors and diabetic ketoacidosis. Endocr Pract. 2016;22(6):753-762. [DOI] [PubMed] [Google Scholar]
- 115. Singh Sukhmani, Rushakoff Robert J., Neinstein Aaron B. A case report of diabetic ketoacidosis with combined use of a sodium glucose transporter 2 inhibitor and hybrid closed-loop insulin delivery. J Diabetes Sci Technol. 2019;13(3):605-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Ogawa W, Sakaguchi K. Euglycemic diabetic ketoacidosis induced by SGLT2 inhibitors: possible mechanism and contributing factors. J Diabetes Investig. 2016;7(2):135-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Cahill GF., Jr. Fuel metabolism in starvation. Annu Rev Nutr. 2006;26:1-22. [DOI] [PubMed] [Google Scholar]
- 118. Rosenstock J, Ferrannini E. Euglycemic diabetic ketoacidosis: a predictable, detectable, and preventable safety concern with sglt2 inhibitors. Diabetes Care. 2015;38(9):1638-1642. [DOI] [PubMed] [Google Scholar]
- 119. Herring RA, Shojaee-Moradie F, Garesse R, et al. Metabolic effects of an SGLT2 inhibitor (Dapagliflozin) during a period of acute insulin withdrawal and development of ketoacidosis in people with type 1 diabetes. Diabetes Care. 2020;43(9):2128-2136. [DOI] [PubMed] [Google Scholar]
- 120. Peters AL, Henry RR, Thakkar P, Tong C, Alba M. Diabetic ketoacidosis with canagliflozin, a sodium-glucose cotransporter 2 inhibitor, in patients with type 1 diabetes. Diabetes Care. 2016;39(4):532-538. [DOI] [PubMed] [Google Scholar]
- 121. Langin D. Adipose tissue lipolysis as a metabolic pathway to define pharmacological strategies against obesity and the metabolic syndrome. Pharmacol Res. 2006;53(6):482-491. [DOI] [PubMed] [Google Scholar]
- 122. Cifuentes M, Albala C, Rojas CV. Differences in lipogenesis and lipolysis in obese and non-obese adult human adipocytes. Biol Res. 2008;41(2):197-204. [PubMed] [Google Scholar]
- 123. Peters AL, McGuire DK, Danne T, et al. Diabetic ketoacidosis and related events with sotagliflozin added to insulin in adults with type 1 diabetes: a pooled analysis of the intandem 1 and 2 studies. Diabetes Care. 2020;43(11):2713-2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Mittendorfer B, Horowitz JF, Klein S. Gender differences in lipid and glucose kinetics during short-term fasting. Am J Physiol Endocrinol Metab. 2001;281(6):E1333-E1339. [DOI] [PubMed] [Google Scholar]
- 125. Goldenberg RM, Gilbert JD, Hramiak IM, Woo VC, Zinman B. Sodium-glucose co-transporter inhibitors, their role in type 1 diabetes treatment and a risk mitigation strategy for preventing diabetic ketoacidosis: the STOP DKA protocol. Diabetes Obes Metab. 2019;21:2192-2202. [DOI] [PubMed] [Google Scholar]
- 126. Evans M, Hicks D, Patel D, Patel V, McEwan P, Dashora U. Optimising the benefits of SGLT2 inhibitors for type 1 diabetes. Diabetes Ther. 2020;11(1):37-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Alva S, Castorino K, Cho H, Ou J. Feasibility of continuous ketone monitoring in subcutaneous tissue using a ketone sensor. J Diabetes Sci Technol. 2021;15(4):768-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Danne T, Garg S, Peters AL, et al. International consensus on risk management of diabetic ketoacidosis in patients with type 1 diabetes treated with sodium-glucose cotransporter (SGLT) inhibitors. Diabetes Care. 2019;42(6):1147-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Garg SK, Peters AL, Buse JB, Danne T. Strategy for mitigating DKA risk in patients with type 1 diabetes on adjunctive treatment with SGLT inhibitors: a STICH protocol. Diabetes Technol Ther. 2018;20(9):572-575. [DOI] [PubMed] [Google Scholar]


