Figure 1.
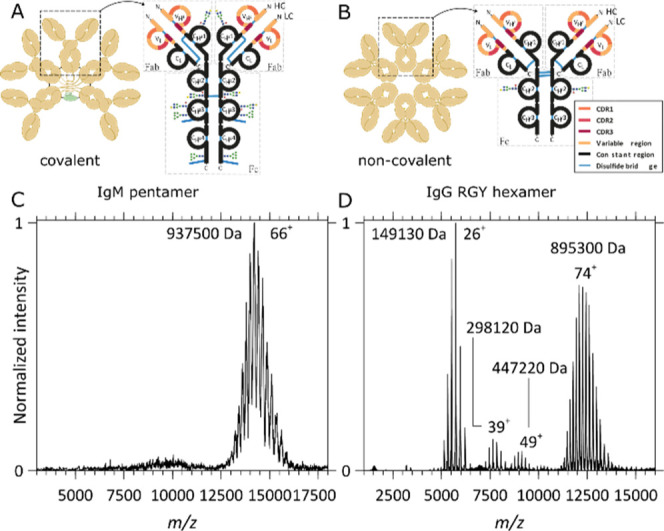
Schematic structures and native MS1 spectra of oligomeric immunoglobulins. (A,C) Structure and native MS1 spectrum of the aWTA IgM pentamer with the J-chain, respectively, and (B,D) structure and native MS1 spectrum of the aCD52 IgG1-RGY hexamer, respectively. While, in (IgM)5J, the IgM’s monomers and J-chain are connected by interchain disulfide bonds, the IgG1 monomers forming the IgG1 hexamers assemble noncovalently. (C) The MS1 spectrum of the aWTA IgM displays a single charge distribution with charge state broadening resulting from the presence of multiple heterogeneous glycans on each monomer. Although heterogeneity and glycan lability hamper an accurate mass analysis, a mean average mass of 937,500 Da could be extracted from this high-resolution data set. (D) In the MS1 spectrum of the aCD52 IgG1-RGY, monomers, dimers, and trimers co-occur with the hexamers, a direct consequence of the noncovalent interactions stabilizing the assembly and of the dynamical equilibria taking place in solution. An average mass of 895,300 Da could be extracted from these data for the hexamer, indeed being within the experimental error 6 times that of the monomer mass.
