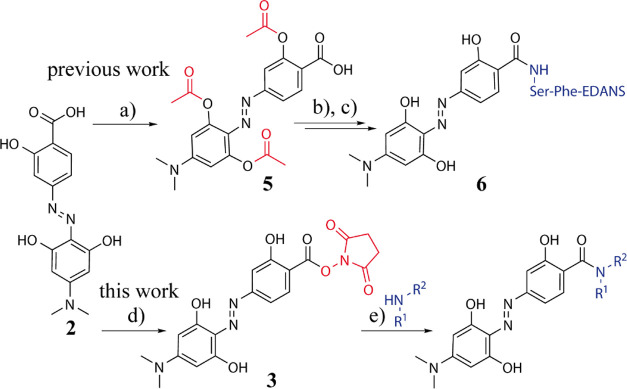
Scheme 1. Strategies for the Coupling of Hydrodabcyl (2) to Amines Either via Acetyl Protection of the Phenolic OH Groups (Marked in Red) in 5 and In Situ Activation of the Carboxylic Acid to Obtain 6 [Previous Work (a–c)] or via Isolation of N-Succinimidyl (Marked in Red) Active Ester 3 to Obtain a Labeled Compound [This Work (d,e)].
Previous work: (a) 4.2 equiv. Ac2O, 6 equiv. NEt3, 0.1 equiv. DMAP, THF/DMSO; 80%. (b) Ser-Phe-EDANS, dipeptide labeled on the Phe-carboxy group as amide with 5-(2-aminoethylamino)-1-naphthalenesulfonic acid (EDANS), 1.6 equiv. propylphosphonic anhydride (T3P), 50% in THF, 3.3 equiv. NEt3, THF/DMF, 0 °C, 1.5 h. (c) piperidine/MeOH, r.t., 1 h; 30% yield over the two steps. This work: (d) 1 equiv. NHS, 1 equiv. CDI, DMF, r.t., overnight; 44%. (e) 1–2 equiv. amine (primary or secondary, HNR1R2), 2 equiv. NEt3, DMF, r.t., overnight; 70–100%. More details are provided in the Supporting Information.