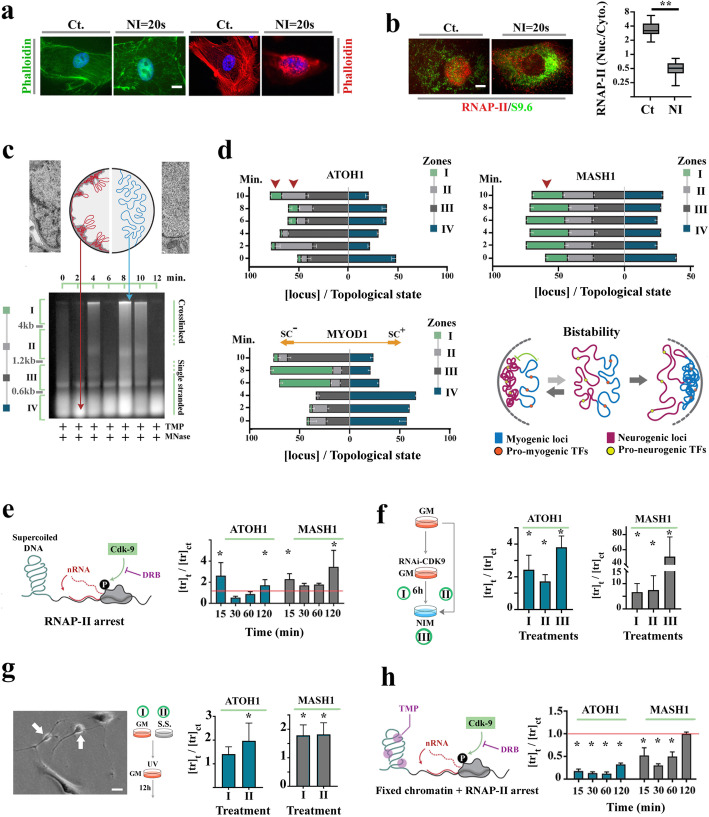
Fig. 2.
Altered higher-order chromatin topology destabilizes the lineage memory of pericytes. a Micrographs show appearance of nuclear Phalloidin+ F-actin (left: fluorescent image, right: Confocal optical slice) after 20 s of exposure to neural induction medium (NI). Scale bar 10 μm. b Immunohistochemical detection of RNAP-II CTD shows depletion of the nuclear complex after 20 s of exposure to neural induction medium (NI). Enhanced cytoplasmic S9.6 immunoreactivity (RNA-DNA hybrid reporter) was consistent with RNAP-II inhibition and subsequent removal. Scale bar 5 μm. Box plot shows the ratio of nuclear to cytoplasmic red signal (RNAP-II) in control and transdifferentiating pericytes (**p < 0.01). c Agarose gel shows oscillations of the higher-order chromatin topology from sc+ to sc− state and vice versa at a frequency of ≈2 min. The isolated chromatin was fixed and fragmented by TMP and MNase, respectively. Chloroquine (5 μg/mL) was used as an interchelator to improve the resolution of 1D gel. DNA was extracted from zones I–IV and probed for the loci of interest using qPCR. d Normalized distribution of loci of interest in various topological states (zones I–IV) that form a gradient from sc−-dominated zone I, with low electrophoretic mobility, to sc+-dominated zone IV, with high electrophoretic mobility. The schematic image shows the proposed model for metastability of pericytes. In the proposed model, global transcriptional landscape not only shapes the functional identity of pericytes but it also represses the competing chromatin state that encodes neural lineage memory. e The graph shows expression of ATOH1 and MASH1 in DRB-treated pericytes normalized to non-treated control pericytes (culture condition: growth medium, *p < 0.01). f The graph shows expression of ATOH1 and MASH1 after RNAi-mediated inhibition of CDK9 in growth medium after 6 h (treatment I) compared to the level of CDK9 after neural induction (treatment II). In treatment III, cells were incubated in neural induction medium after RNAi-mediated inhibition of CDK9 (*p < 0.01). g The graphs show expression of ATOH1 and MASH1 after UVC radiation of cycling pericytes (treatment I) and serum-starved cells (treatment II) followed by incubation in growth medium (*p < 0.01). h The graph shows expression of the same genes in DRB-treated pericytes with a fixed chromatin topology (TMP-treated) normalized to DRB-treated control pericytes with native chromatin (culture condition: growth medium (*p < 0.01)