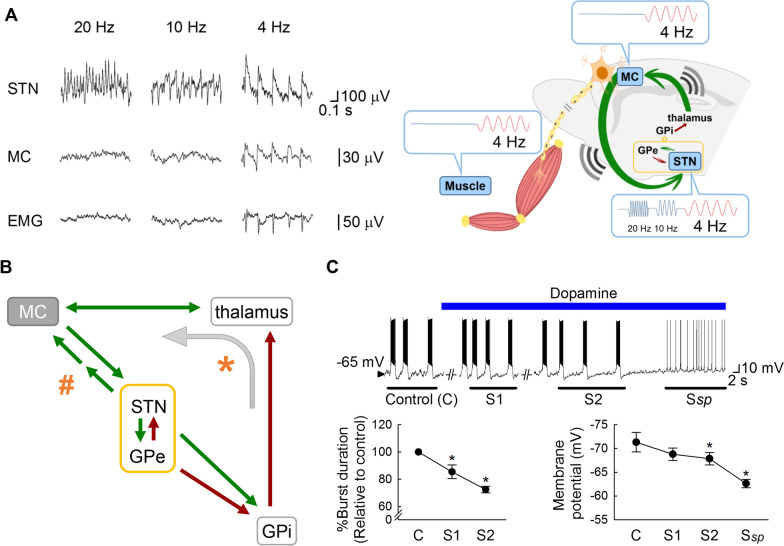
Fig. 3.
Information relay from STN to MC and the oscillating activities in the cortico-subcortical re-entrant loops. A With local application of 4-aminopyridine (4-AP), prominent repetitive burst discharges and oscillatory activities in LFP develop in STN at ~ 20, 10, or 4 Hz. However, coherent LFP oscillations in MC with vivid tremulous movement (muscle activities detected by electromyography or EMG) of the same frequencies could be discerned only with the ~ 4 Hz, but not ~ 10 or 20 Hz oscillating bursts (left, recording traces; right, a schematic drawing; also see [50]). This demonstrates that ~ 4 but not ~ 10 or 20 Hz is within the entrainable intrinsic frequency range of the major elements of the cortico-subcortical re-entrant loops, so that “resonance” of the major elements, including STN and MC, may ensue. Recordings were from an anesthetized 2-month-old parkinsonian (6-OHDA-lesioned) male Wistar rat. B A schematic drawing for possible major connections between MC and STN (green arrows: glutamatergic pathways; crimson arrows: GABAergic pathways). In contrast to the hyperdirect (corticosubthalamic) pathway which conveys MC drives directly to STN, there is no well-established direct input from STN to MC [123]. There are, then, two possible ways for the relay of information from STN to MC (as in part A). Indirect excitatory pathways via, for example, the pedunculopontine nucleus which has reciprocal connections with both MC and STN [124] and is also a locomotor center for the control of muscle tone [125, 126], may exist to relay the STN bursts back to MC with a phase lag (indicated by # and the two consecutive arrows). Together with the hyperdirect pathway, this may contribute to the necessary extrinsic excitatory drive for continual oscillations in the STN-GPe core. On the other hand, the STN-GPe oscillating activities may travel via GPi (indicated by * and the curve arrow), the main output station of basal ganglia, to implement rhythms to the thalamocortical oscillation circuitry. C In acute brain slices from parkinsonian (6-OHDA-lesioned) male Wistar rats (aged 5–6 weeks, n = 4–5), perfusion of dopamine (25 μM) gradually depolarizes the membrane and shortens the burst discharges in STN neurons until a stable spike mode of discharges (Ssp) appears. The lowest membrane potential and the burst duration is measured and averaged from the three consecutive bursts immediately after the 30th sec of dopamine perfusion (S1) and just before the transition to the spike mode (S2). Data are mean ± SEM. *P < 0.05 compared to control (before dopamine), Friedman tests followed by Wilcoxon signed-rank tests for further pairwise comparison. See Huang et al., 2021 [50] for more experimental details in parts A and C