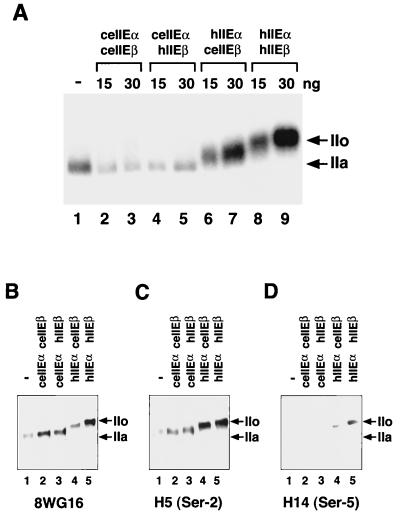
FIG. 7.
Effects of ceTFIIE subunits on CTD phosphorylation by TFIIH during preinitiation complex formation. Kinase assays (25 μl) were carried out as described in Materials and Methods. TFIIE proteins were prepared and purified as described for Fig. 4A and in Materials and Methods. (A) CTD phosphorylation of intact Pol II during PIC formation. Lane 1, kinase reaction without TFIIE (−); lanes 2 and 3, ceTFIIE (ceIIEαceIIEβ); lanes 4 and 5, chimeric TFIIE made up of ceTFIIEα and hTFIIEβ (ceIIEαhIIEβ); lanes 6 and 7, chimeric TFIIE made up of hTFIIEα and ceTFIIEβ (hIIEαceIIEβ); lanes 8 and 9, hTFIIE (hIIEαhIIEβ). For lanes 2, 4, 6, and 8, 15 ng of each TFIIE was added. For lanes 3, 5, 7, and 9, 30 ng of each TFIIE was added. Phosphorylated proteins were analyzed on a 5.5% acrylamide-SDS gel and detected by autoradiography. Arrows indicate the positions of the phosphorylated (IIo) and unphosphorylated (IIa) forms of the largest subunit of Pol II. (B) Detection of the largest subunit of Pol II after treatment with kinases. The kinase reaction was carried out essentially as described for panel A, except that nonisotopic ATP was used instead of [γ-32P]ATP and a monoclonal antibody (8WG16) against the CTD heptapeptide was used to detect the largest subunit of Pol II. (C) Detection of phosphorylated Ser-2 in the CTD of the largest subunit of Pol II. Reactions were carried out as described for panel B, and phospho-Ser-2 in the CTD was detected using the monoclonal antibody H5. (D) Detection of phosphorylated Ser-5 in the CTD of the largest subunit of Pol II. Reactions were carried out as described for panel B, and phospho-Ser-5 in the CTD was detected using the monoclonal antibody H14.