Fig. 2 |. Cross-domain impact of RGDs and limitations of current evidence.
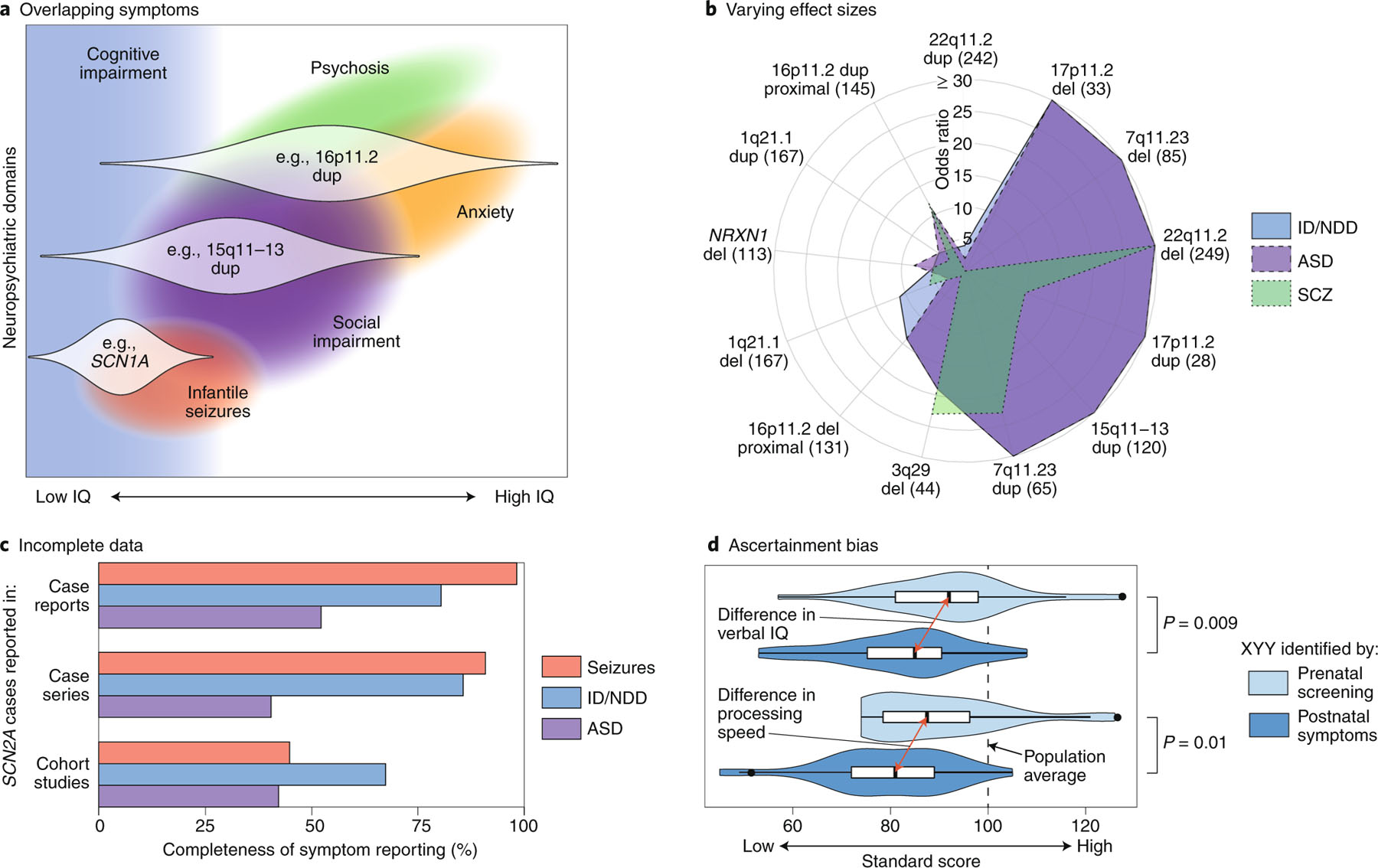
a, A theoretical model of how three RGDs (16p11.2 dup, 15q11–13 dup and SCN1A) impact multiple neuropsychiatric domains across different individuals (distribution across affected individuals shown as white violin plots)34,127,128. b, A polar plot showing the varying effect sizes (odds ratios) of different CNVs on the diagnosis of ID/NDD, ASD and SCZ (Supplementary Table 2, Extended Data Fig. 1). The number of CNV cases are shown in parentheses5,8,32,129–131; the UK Biobank was used for controls33. c, The completeness of symptom reporting for SCN2A mutations varies widely between publications132. Case reports describe a single SCN2A mutation in one case or family; case series describe multiple SCN2A cases; cohort studies describe hundreds of cases with the same disorder (for example, ID/NDD), some of which are found to have SCN2A mutations. This reporting bias, which is likely to be present for most RGDs, complicates comparisons across neuropsychiatric domains and between RGDs. d, The severity of symptoms in XYY aneuploidy varies between cases ascertained by prenatal screening (light blue) and those ascertained on the basis of clinical symptoms (dark blue)133. This ascertainment bias, which is also likely to be present for most RGDs, also complicates cross-disorder comparisons and potentially inflates estimates of effect size and penetrance. ID, intellectual disability; NDD, neurodevelopmental delay; ASD, autism spectrum disorder; SCZ, schizophrenia; IE: infantile epilepsy; CNV, copy-number variant. Credit: Debbie Maizels/Springer Nature.
