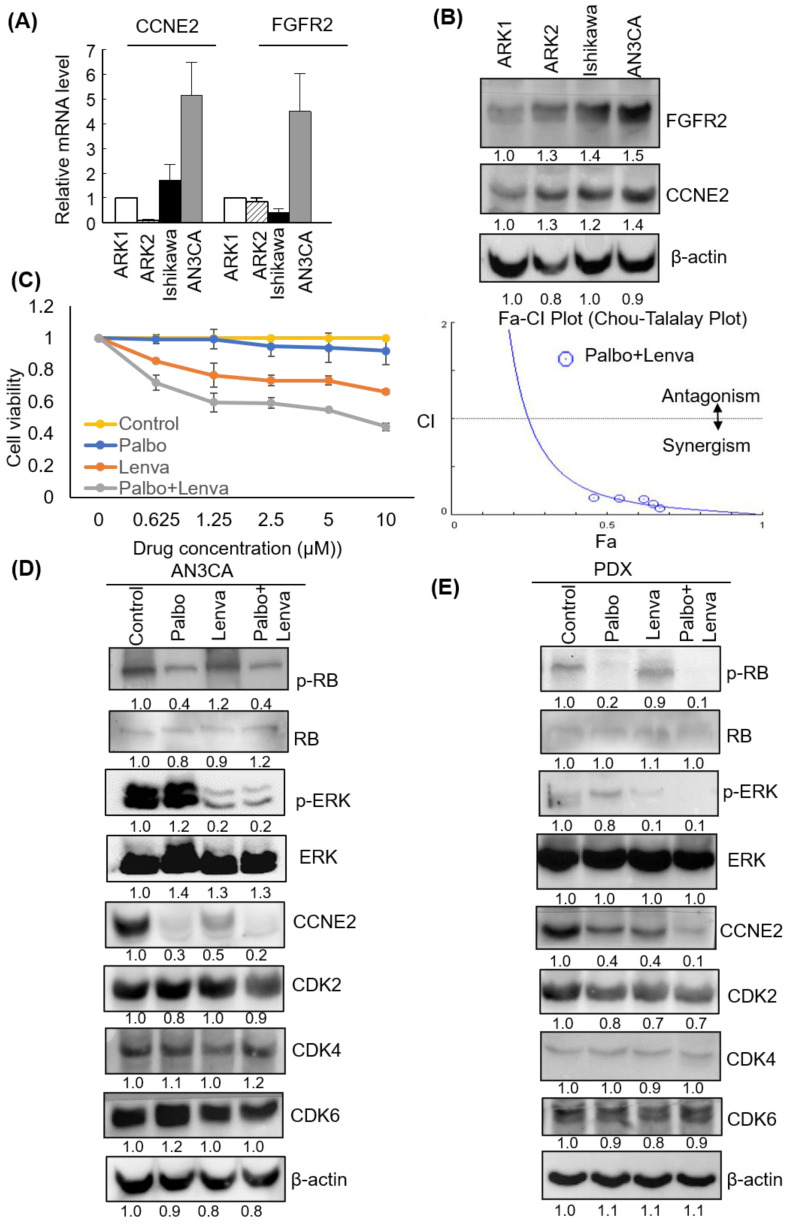
Figure 5.
Palbociclib and lenvatinib synergistically inhibited CCNE2 expression levels and decreased viability of endometrial cancer (EC) cells. CCNE2 and FGFR2 (A) mRNA and (B) protein expression in ARK1, ARK2, Ishikawa, and AN3CA cell lines, respectively. β-actin served as control for normalization. The numbers below the data mean densitometry-derived values normalized to ARK1 (set to 1). β-actin served as loading control for normalization. (C) AN3CA cells were treated for 48 h with vehicle (−) or different doses of palbociclib alone (0.625, 1.25, 2.5, 5, and 10 μM), lenvatinib alone (0.625, 1.25, 2.5, 5, and 10 μM), or their combination. Cell survival was analyzed with the MTT assay. Data from three different experiments (each performed in triplicate) are expressed as fold changes ± standard deviations relative to vehicle-treated cells (left panel). The synergistic effects of palbociclib and lenvatinib were analyzed using the CompuSyn software (right. panel). (D) Palbociclib (10 μM), lenvatinib (10 μM) and their combination were used to treat AN3CA cells for 48 h. (E) Tissues from the PDX-mlung model treated with palbociclib, lenvatinib, or their combination were collected. Cell lysates were subsequently resolved on SDS-PAGE and subjected to immunoblotting with antibodies raised against p-RB, RB, p-ERK1/2, ERK1/2, CCNE2, CDK2, CDK4, CDK6, and β-actin. The numbers below the data mean densitometry-derived values normalized to control (set to 1). β-actin served as loading control for normalization. Abbreviations: Palbo, palbociclib; Lenva, lenvatinib; Palbo+Lenva: palbociclib and lenvatinib given in combination.