FIG. 6.
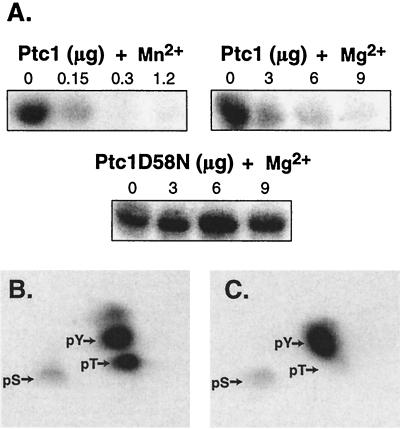
Ptc1 inactivates Hog1 kinase activity in vitro by dephosphorylating pT. (A) Ptc1 inactivated Hog1 kinase activity in vitro. GST-Hog1 was isolated from osmotically stressed yeast strain JWY1 and incubated in the absence or presence of wild-type Ptc1 or mutant Ptc1D58N for 30 min at 30°C. Ptc1 or PtcD58N in the amounts specified (0 to 9 μg) was incubated in the presence of 5 mM Mg2+ or 20 mM Mn2+. The bead-bound GST-Hog1 was washed extensively and incubated with MBP and [γ-32P]ATP. Radiolabel incorporated into MBP was examined by PhosphorImager analysis. (B) Hog1 was phosphorylated at Thr and Tyr by the hyperactive MEK mutant, Pbs2EE, in vitro. GST-Hog1 was isolated from untreated yeast using glutathione-Sepharose. The bead-bound GST-Hog1 was phosphorylated by incubation with Pbs2EE purified from E. coli and [γ-32P]ATP. Phosphoamino acid analysis was performed to examine the level of pT and pY. Arrows indicate the position of phosphoamino acid standards as revealed by ninhydrin staining. pS, phosphoserine. (C) Ptc1 specifically dephosphorylates Hog1-pThr in vitro. 32P-phosphorylated GST-Hog1 was treated with Ptc1 and examined as above.